Sandbox Reserved 1852
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
==Introduction== | ==Introduction== | ||
| + | Å | ||
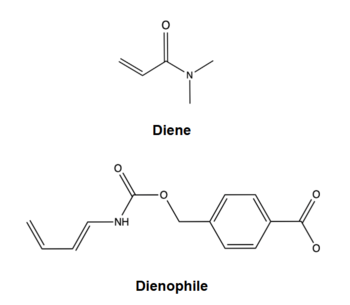
The Diels Alderase aims to catalyze the Diels-Alder reaction for use in synthetic organic chemistry. Specifically, the enzyme surpasses uncatalyzed reactions by generating a product that is entirely [https://en.wikipedia.org/wiki/Stereoselectivity#:~:text=In%20chemistry%2C%20stereoselectivity%20is%20the,of%20a%20pre%2Dexisting%20one. stereoselective] for the 3R,4S endo form. The Diels-Alderase was built using ''de novo'' enzyme design, using computational modeling and refinement through collaborative problem-solving from online users. The first generation Diels-Alderase was made using the [https://en.wikipedia.org/wiki/Rosetta@home Rosetta] computational design program, where a potential active site was built and tested against a library of scaffold proteins. Later, as the active site was perfected, future generations of the Diels-Alderase were made using an online protein folding game called [https://en.wikipedia.org/wiki/Foldit Foldit,] where players competed to improve binding efficiency by completing various challenges.<ref name="Eiben"/> | The Diels Alderase aims to catalyze the Diels-Alder reaction for use in synthetic organic chemistry. Specifically, the enzyme surpasses uncatalyzed reactions by generating a product that is entirely [https://en.wikipedia.org/wiki/Stereoselectivity#:~:text=In%20chemistry%2C%20stereoselectivity%20is%20the,of%20a%20pre%2Dexisting%20one. stereoselective] for the 3R,4S endo form. The Diels-Alderase was built using ''de novo'' enzyme design, using computational modeling and refinement through collaborative problem-solving from online users. The first generation Diels-Alderase was made using the [https://en.wikipedia.org/wiki/Rosetta@home Rosetta] computational design program, where a potential active site was built and tested against a library of scaffold proteins. Later, as the active site was perfected, future generations of the Diels-Alderase were made using an online protein folding game called [https://en.wikipedia.org/wiki/Foldit Foldit,] where players competed to improve binding efficiency by completing various challenges.<ref name="Eiben"/> | ||
| Line 27: | Line 28: | ||
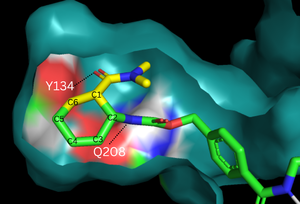
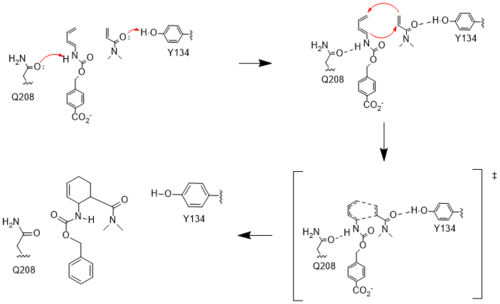
The two active site residues, Y134 and Q208, use hydrogen bonding to close the energy gap between the [https://en.wikipedia.org/wiki/Diels%E2%80%93Alder_reaction HOMO diene and the LUMO dienophile]. The goal of closing the energy gap allows the diene and dienophile to readily switch roles for the mechanism to progress and complete the formation of the product. Due to the conserved nature of this mechanism, the diels-alderase is stereoselective for the 3R, 4S endo product. | The two active site residues, Y134 and Q208, use hydrogen bonding to close the energy gap between the [https://en.wikipedia.org/wiki/Diels%E2%80%93Alder_reaction HOMO diene and the LUMO dienophile]. The goal of closing the energy gap allows the diene and dienophile to readily switch roles for the mechanism to progress and complete the formation of the product. Due to the conserved nature of this mechanism, the diels-alderase is stereoselective for the 3R, 4S endo product. | ||
====Hydrogen Bonding==== | ====Hydrogen Bonding==== | ||
| - | Rather than using [https://en.wikipedia.org/wiki/Acid_catalysis acid-base catalysis] | + | Rather than using [https://en.wikipedia.org/wiki/Acid_catalysis acid-base catalysis], the Diels-Alderase utilizes hydrogen bonding to alter the HOMO and LUMO energies of the diene and dienophile. Tyr134 donates a hydrogen bond to the dieneophile, increasing its electron density and lowering its LUMO. Glu208 accepts a hydrogen bond from the diene, decreasing its electron density and lowering its HOMO. |
| Line 40: | Line 41: | ||
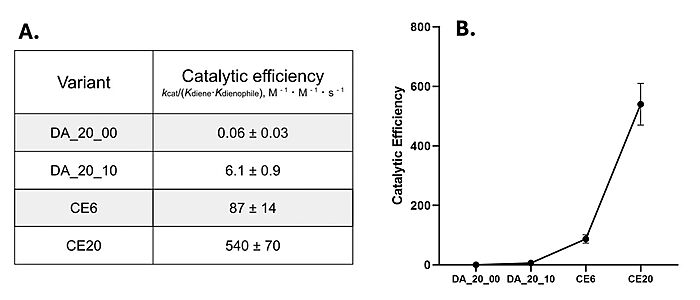
DA_20_10 provided key mutations in and around the active site that increased the hydrophobicity, provided structural stability, and increased interactions between the ligand and surrounding residues. | DA_20_10 provided key mutations in and around the active site that increased the hydrophobicity, provided structural stability, and increased interactions between the ligand and surrounding residues. | ||
=====Q162R===== | =====Q162R===== | ||
| - | :Glu 162, a <scene name='10/1075254/Q162/2'>glutamine</scene>, resides near the top of the binding site | + | :Glu 162, a <scene name='10/1075254/Q162/2'>glutamine</scene>, resides near the top of the binding site, and is more than 3Å from the ligand in most models on the enzyme. It can act as a hydrogen bond donor to the terminal phosphate on the ligand when in proximity. To increase this interaction, Glu162 was mutated to an <scene name='10/1075254/Q_to_r/1'>arginine</scene>, which decreased the length of the potential hydrogen bond to within 2.5Å, increasing the strength of the interaction.<ref name="Siegel"/> |
=====S284A===== | =====S284A===== | ||
:Ser 284 resides deep within the binding pocket of the enzyme. Choosing a <scene name='10/1075253/S284/2'>serine</scene> to <scene name='10/1075253/A285_scence/2'>alanine</scene> mutation increases the hydrophobicity of the binding pocket and reduce reactivity, without also changing any steric characteristics in the region ''unintentionally'' near the catalytic residues.<ref name="Siegel"/> | :Ser 284 resides deep within the binding pocket of the enzyme. Choosing a <scene name='10/1075253/S284/2'>serine</scene> to <scene name='10/1075253/A285_scence/2'>alanine</scene> mutation increases the hydrophobicity of the binding pocket and reduce reactivity, without also changing any steric characteristics in the region ''unintentionally'' near the catalytic residues.<ref name="Siegel"/> | ||
Revision as of 19:54, 17 April 2025
| This Sandbox is Reserved from March 18 through September 1, 2025 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson and Mark Macbeth at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1828 through Sandbox Reserved 1846. |
To get started:
More help: Help:Editing |
Diels-Alderase
| |||||||||||