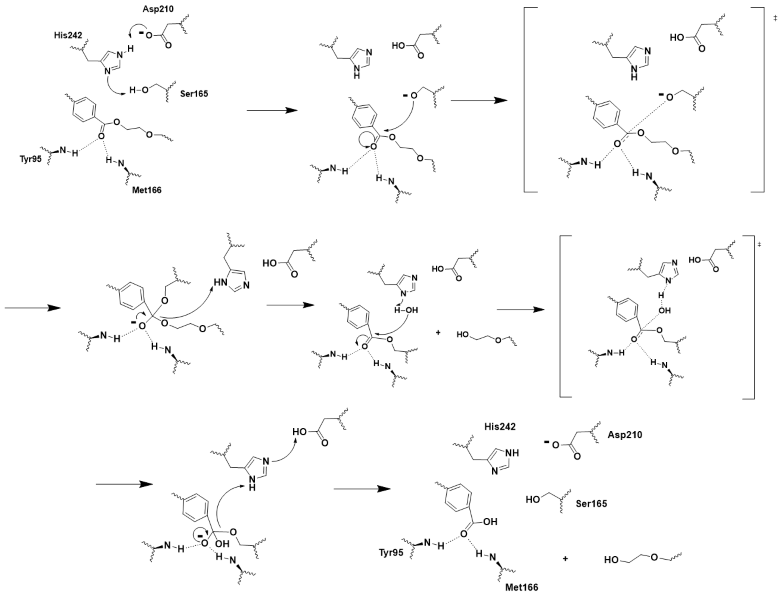
User:Hayden Vissing/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 31: | Line 31: | ||
===Efficiency improvement=== | ===Efficiency improvement=== | ||
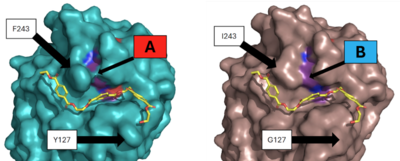
[[Image:Thermo_pic.png|400px|right|thumb|Figure 3 - Protein surface images displaying the impact on the active site]] | [[Image:Thermo_pic.png|400px|right|thumb|Figure 3 - Protein surface images displaying the impact on the active site]] | ||
| - | Two of the mutations were selected as they showed the best improvement in catalytic efficiency. The mutation of residue 127 from a <scene name='10/1076051/Y127/2'>tyrosine</scene> to a <scene name='10/1075218/G127/6'>glycine</scene> and the mutation of residue 243 from a <scene name='10/1076051/F243/4'>phenylalanine</scene> to an <scene name='10/1075218/I243/3'>isoleucine</scene> improved the specific activity of the PET depolymerization of the enzyme by around 27%<ref name="main" />. This increase in catalytic activity was likely due to the decreased average distance between the catalytic residues in the mutant enzyme. This change can be seen in the surface view of the enzyme’s active site, which shows the loss of the “bridge” over the active site created by the interaction between F243 and its nearby residues. The loss of these interactions when changed to I243 most likely allows for the relaxation of the active site residues and the decrease in the distance between them | + | Two of the mutations were selected as they showed the best improvement in catalytic efficiency. The mutation of residue 127 from a <scene name='10/1076051/Y127/2'>tyrosine</scene> to a <scene name='10/1075218/G127/6'>glycine</scene> and the mutation of residue 243 from a <scene name='10/1076051/F243/4'>phenylalanine</scene> to an <scene name='10/1075218/I243/3'>isoleucine</scene> improved the specific activity of the PET depolymerization of the enzyme by around 27%. Molecular simulations showed that the distance between the carbonyl carbon of the substrate, oxygen of S165, and the nitrogen of H242 was decreased<ref name="main" />. This increase in catalytic activity was likely due to the decreased average distance between the catalytic residues in the mutant enzyme. This change can be seen in the surface view of the enzyme’s active site, which shows the loss of the “bridge” over the active site created by the interaction between F243 and its nearby residues. The loss of these interactions when changed to I243 most likely allows for the relaxation of the active site residues and the decrease in the distance between them. |
===Thermostability improvement=== | ===Thermostability improvement=== | ||
| Line 39: | Line 39: | ||
===Future directions=== | ===Future directions=== | ||
| + | The development of the ICCG PET Hydrolase enzyme still has room for improvement. To start, future mutagenesis can be conducted to see if the efficiency of the enzyme can be improved further. Some possible modifications could be A59K, V63I, or N248P.<ref name="Hydrolases" /> ICCG isn’t the only enzyme that is available for modification either, other known PET Hydrolase enzymes, such as IsPETase, could be modified by a similar mutagenesis process.<ref name="Hydrolases" /> Other degradation processes could be examined to determine their effectiveness in degrading PET. For example, glycolysis could be the next type of degradation to explore. It involves the breakdown of PET in the presence of glycols using metal-oxide based catalysts and can be performed at lower temperatures than hydrolysis.<ref name="main" /> Finally, it could also be possible to use this mutagenesis process to improve the ability of other hydrolases to break down different classes of plastics.<ref name="Hydrolases" />. | ||
</StructureSection> | </StructureSection> | ||
Revision as of 19:24, 22 April 2025
The Future of Recycling: the ICCG Mutant Pet Hydroalase
| |||||||||||
References
- ↑ Hiraga, K., Taniguchi, I., Yoshida, S., Kimura, Y., & Oda, K. (2019). Biodegradation of waste PET: A sustainable solution for dealing with plastic pollution. EMBO Reports, 20(11), e49365. https://doi.org/10.15252/embr.201949365. [Published correction appears in EMBO Reports, 21(2), e49826. DOI: 10.15252/embr.201949826
- ↑ Jayasekara, S. K., Joni, H. D., Jayantha, B., Dissanayake, L., Mandrell, C., Sinharage, M. M. S., Molitor, R., Jayasekara, T., Sivakumar, P., & Jayakody, L. N. (2023). Trends in in-silico guided engineering of efficient polyethylene terephthalate (PET) hydrolyzing enzymes to enable bio-recycling and upcycling of PET. Computational and structural biotechnology journal, 21, 3513–3521. DOI: 10.1016/j.csbj.2023.06.004
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Tournier V, Topham CM, Gilles A, David B, Folgoas C, Moya-Leclair E, Kamionka E, Desrousseaux ML, Texier H, Gavalda S, Cot M, Guemard E, Dalibey M, Nomme J, Cioci G, Barbe S, Chateau M, Andre I, Duquesne S, Marty A. An engineered PET depolymerase to break down and recycle plastic bottles. Nature. 2020 Apr;580(7802):216-219. doi: 10.1038/s41586-020-2149-4. Epub 2020 Apr, 8. PMID:32269349 doi:http://dx.doi.org/10.1038/s41586-020-2149-4
- ↑ Joo S, Cho IJ, Seo H, Son HF, Sagong HY, Shin TJ, Choi SY, Lee SY, Kim KJ. Structural insight into molecular mechanism of poly(ethylene terephthalate) degradation. Nat Commun. 2018 Jan 26;9(1):382. doi: 10.1038/s41467-018-02881-1. PMID:29374183 doi:http://dx.doi.org/10.1038/s41467-018-02881-1
- ↑ Sulaiman S, Yamato S, Kanaya E, Kim JJ, Koga Y, Takano K, Kanaya S. Isolation of a novel cutinase homolog with polyethylene terephthalate-degrading activity from leaf-branch compost by using a metagenomic approach. Appl Environ Microbiol. 2012 Mar;78(5):1556-62. doi: 10.1128/AEM.06725-11. Epub, 2011 Dec 22. PMID:22194294 doi:http://dx.doi.org/10.1128/AEM.06725-11
- ↑ Han, X., Liu, W., Huang, J. W., et al. (2017). Structural insight into catalytic mechanism of PET hydrolase. Nature Communications, 8, 2106. https://doi.org/10.1038/s41467-017-02255-z.Heredia-Guerrero, J. A., Heredia, A., García-Segura, R., & Benítez, J. J. (2009). Synthesis and characterization of a plant cutin mimetic polymer. Polymer, 50(24), 5633–5637. DOI: 10.1016/j.polymer.2009.10.018
- ↑ A binding model of the substrate 2-HE(MHET)3 in wild-type LLC (4eb0.pdb) was constructed and refined to mimic the 3D structure illustrated in Figure 2 of reference “3”. The software Maestro (Schrödinger, Inc; version 14.2.118) was used to construct the initial binding structure, followed by energy minimization in the context of the rigid protein that had previously been processed to add/refine all hydrogen atoms. The ligand model was then used without further modification to identify and illustrate the cited active-site residues.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedHydrolases
Additional Literature and Resources
- Babaei, M., Jalilian, M., & Shahbaz, K. (2024). Chemical recycling of Polyethylene terephthalate: A mini-review. Journal of Environmental Chemical Engineering, 12(3), 112507. DOI: 10.1016/j.jece.2024.112507
- Roth C, Wei R, Oeser T, Then J, Follner C, Zimmermann W, Strater N. Structural and functional studies on a thermostable polyethylene terephthalate degrading hydrolase from Thermobifida fusca. Appl Microbiol Biotechnol. 2014 Apr 13. PMID:24728714 doi:http://dx.doi.org/10.1007/s00253-014-5672-0
Student Contributors
- David Bogle
- Justin Chavez
- Hayden Vissing