Introduction
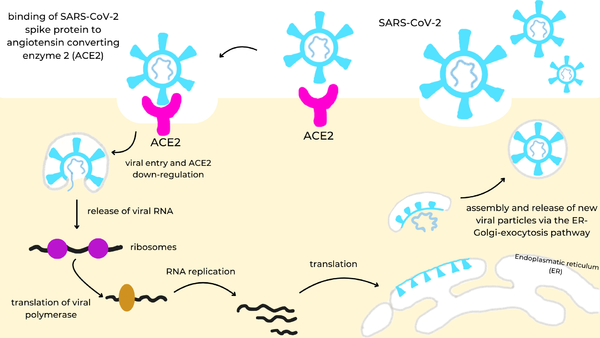
SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) is an RNA virus that is responsible for the highly infectious respiratory disease, COVID-19.
Overall SARS-CoV-2 Spike Structure
It is made up of four structural proteins: spike, envelope, membrane, and nucleocapsid proteins. The spike protein helps the virus infect human cells. The envelope protein helps in virus assembly and release. The membrane protein shapes the viral envelope. The nucleocapsid protein binds the RNA genome for replication. Once inside the cell, virus-specific RNA and proteins are synthesized within the cytoplasm. The viral envelope merges with the oily membrane of our own cells, allowing the virus to release its genetic material into the inside of the healthy cell. The genetic blueprint of the virus is RNA instead of DNA so it gives infected host cells instructions to read the template and translate it to replicate the virus. Each infected cell may produce and release millions of copies of the virus which can infect other neighboring cells and people when the viral particles are released from the airways (i.e., via coughing or sneezing).
Spike Protein
The spike protein[1] is a glycoprotein that lies on the surface of SARS-CoV-2 to facilitate the entry of the virus into host cells. More specifically, once SARS-CoV-2 has entered the respiratory system, its spike protein binds to the ACE2 receptor (Angiotensin Converting Enzyme Receptor 2) in the lungs. The spike protein is cleaved into two functional subunits: S1 and S2. S1 contains the RBDs (receptor binding domains). S2 plays a key role in membrane fusion between the virus and host cell, allowing viral RNA to enter.
Receptor Binding Domains
The spike protein is a homotrimer with three Receptor Binding Domains , containing the binding site. The spike protein’s RBD is the region where it attaches to the human ACE2 receptor. A at the RBDs reveal a mostly beta-sheet secondary structure with a handful of helices scattered about. There are also a couple variable loops where a number of interactions take place as well. These interactions are specific to the binding ligand and will be talked about in depth later.
ACE2 Receptor
the natural receptor for the spike protein, is a membrane-bound protein located in the lungs and made up of 805 amino acids. Its is located within a single extracellular N-terminal domain. Residues 26-43 in this region form an alpha helix which makes the majority of interactions with the RBD of the spike protein. These interactions are stabilized by hydrogen bonding[2] and van der Waals forces[3].
Mini Protein Inhibitors
AHB2
is a stable miniprotein inhibitor that competes with the ACE2 receptor. It was developed using the Rosetta blueprint builder that incorporates the alpha helix in ACE2 (residues 23-46) into small, stable protein scaffolds. This molecule utilizes van der Waals interactions in its to stabilize. show an increased number of interactions between the spike and minibinder. This allows it to compete with the ACE2 receptor more effectively, preventing it from binding with the spike protein.[1]
LCBs
is a fully de novo three-helix mini protein that acts as a competitive inhibitor, competing with ACE2 to bind to the spike protein. Unlike ABH2, a de novo design approach was taken to design LCB1, making it more compact with optimal interactions with the RBD. Its show a large number of hydrogen bonds as well as a small hydrophobic effect promoting binding even more so.
Another mini protein inhibitor, was developed using the same computational method as LCB1 and binds to the same region of the RBD, except in the opposite direction. Its also show a large number of hydrogen bonds. However, LCB1 has more surface area and fits more precisely with its computational model, giving it a slightly higher binding affinity.
The LCBs have a significantly higher affinity for the spike RBD and are smaller and more stable than traditional antibodies. Their reduced size allows these molecules to tightly pack more interactions in the active site as well as improve their ability to enter the respiratory system.
Significance
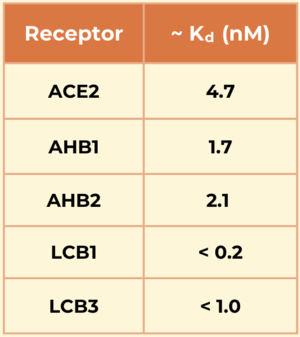
The designed mini protein inhibitors have a significantly higher affinity for the spike protein compared to the ACE2 receptor, especially the LCBs.
Function
[2]
[3]
[4]
Therapeutic Relevance
[2]
[3]
References
- ↑ Cao L, Goreshnik I, Coventry B, Case JB, Miller L, Kozodoy L, Chen RE, Carter L, Walls AC, Park YJ, Strauch EM, Stewart L, Diamond MS, Veesler D, Baker D. De novo design of picomolar SARS-CoV-2 miniprotein inhibitors. Science. 2020 Oct 23;370(6515):426-431. PMID:32907861 doi:10.1126/science.abd9909
- ↑ 2.0 2.1 Ransey E, Paredes E, Dey SK, Das SR, Heroux A, Macbeth MR. Crystal structure of the Entamoeba histolytica RNA lariat debranching enzyme EhDbr1 reveals a catalytic Zn(2+) /Mn(2+) heterobinucleation. FEBS Lett. 2017 Jul;591(13):2003-2010. doi: 10.1002/1873-3468.12677. Epub 2017, Jun 14. PMID:28504306 doi:http://dx.doi.org/10.1002/1873-3468.12677
- ↑ 3.0 3.1 Gusev E, Sarapultsev A, Solomatina L, Chereshnev V. SARS-CoV-2-Specific Immune Response and the Pathogenesis of COVID-19. Int J Mol Sci. 2022 Feb 2;23(3):1716. PMID:35163638 doi:10.3390/ijms23031716
- ↑ Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020 Mar 30. pii: 10.1038/s41586-020-2180-5. doi:, 10.1038/s41586-020-2180-5. PMID:32225176 doi:http://dx.doi.org/10.1038/s41586-020-2180-5
Student Contributors
- Carson Powers
- Rachael Vavul
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.