We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Marcos Ngo/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
<StructureSection load='7rds' size='340' side='right'caption='[[7rds]], [[Resolution|resolution]] 2.50Å' scene=''> | <StructureSection load='7rds' size='340' side='right'caption='[[7rds]], [[Resolution|resolution]] 2.50Å' scene=''> | ||
== Function and Background== | == Function and Background== | ||
| - | DNA glycosylases search the entire genome for DNA lesions. These highly selective enzymes recognize a damaged base and remove it. There are four super families of glycosylases: Udg, Nth, Nei, and AGG. '''hNTHL1''' or '''human Endonuclease III''' (Nth family) is a 34 kDa bifunctional DNA glycosylase involved in the base excision repair (BER) process. A bifunctional glycosylase refers to the ability to '''excise''' damaged bases and '''cleave''' the backbone. | + | DNA glycosylases search the entire genome for DNA lesions. These highly selective enzymes recognize a damaged base and remove it. There are four super families of glycosylases: Udg, Nth, Nei, and AGG. '''hNTHL1''' or '''human Endonuclease III''' (Nth family) is a 34 kDa bifunctional DNA glycosylase involved in the base excision repair (BER) process. A bifunctional glycosylase refers to the ability to '''excise''' damaged bases and '''cleave''' the backbone. hNTHL1 preferentially excises oxidized pyrimidines. Thymine glycol (Tg) is the preferred substrate. Upon encountering this damaged base, the protein severs the N-glycosidic bond, which leaves an apurinic site. From here, the backbone is cleaved via beta elimination, which leaves a 3’ aldehyde and creates a single-strand break. Next, the DNA is handed off to Apurinic endonuclease 1 or polynucleotide kinase, leaving a free 3′ hydroxyl for DNA polymerase β to insert the correct nucleotide. Finally, the nick is sealed by the DNA ligase IIIα <ref>PMID:34871433</ref><ref>PMID:20005182</ref><ref>PMID:9295348</ref>. |
The gene encoding hNTHL1 is NTHL1, which is located on chromosome 16. It is widely expressed across tissues, with the highest levels observed in the heart. This elevated expression may reflect the heart’s high demand for ATP production, which generates significant oxidative stress and thus increases reliance on base excision repair (BER) proteins for genome maintenance. Additionally, hNTHL1 expression is regulated during the cell cycle, with transcription levels rising during the early and mid S phases <ref>https://www.uniprot.org/uniprotkb/P78549/entry</ref><ref>PMID:8990169</ref><ref>PMID:10882850</ref>. | The gene encoding hNTHL1 is NTHL1, which is located on chromosome 16. It is widely expressed across tissues, with the highest levels observed in the heart. This elevated expression may reflect the heart’s high demand for ATP production, which generates significant oxidative stress and thus increases reliance on base excision repair (BER) proteins for genome maintenance. Additionally, hNTHL1 expression is regulated during the cell cycle, with transcription levels rising during the early and mid S phases <ref>https://www.uniprot.org/uniprotkb/P78549/entry</ref><ref>PMID:8990169</ref><ref>PMID:10882850</ref>. | ||
| Line 10: | Line 10: | ||
== Mechanism and Repair == | == Mechanism and Repair == | ||
| - | DNA glycosylases | + | DNA glycosylases remove damaged bases through a “pinch, push, plug, and pull” mechanism. First, the DNA is “pinched” by the enzyme, which destabilizes the helix. Next, they use a wedge amino acid to “push” the lesion out of the helix. While the lesion is being flipped out, another amino acid “plugs” into the helix to fill the gap and maintain the structure of the helix. Finally, the lesion is “pulled” into the active site to allow for lesion removal <ref>https://scholarworks.uvm.edu/cgi/viewcontent.cgi?article=2160&context=graddis</ref><ref>PMID:20469926</ref><ref>PMID:12220189</ref>. |
| - | Recent studies have investigated how hNTH1 initiates BER of oxidative lesions in nucleosomes, where DNA is wrapped around a histone core. When lesions are away from the histone core, hNTHL1 | + | Recent studies have investigated how hNTH1 initiates BER of oxidative lesions in nucleosomes, where DNA is wrapped around a histone core. When lesions are positioned away from the histone core, hNTHL1 processes them with nearly the same efficiency as in naked DNA (without histones). When lesions faced inward toward the histone core, repair was initially poor but improved significantly at higher hNTHL1 concentrations. Additionally, sections near the edge of the nucleosome were repaired more efficiently than those near the nucleosome center. This suggests that both the partial unwrapping of DNA from the histone core and the positioning of the lesion outward from the nucleosome edge allow hNTHL1 to efficiently access and repair DNA lesions <ref>PMID:17923696</ref>. |
== Structural Highlights == | == Structural Highlights == | ||
| Line 29: | Line 29: | ||
The function of the <scene name='10/1077482/N-terminus_AlphaFold/3'>N-terminus</scene> (AlphaFold Prediction) of hNTHL1 has been a subject of study <ref>https://www.nature.com/articles/s41586-021-03819-2/</ref><ref>PMID:37933859</ref>. It is theorized that the N-terminus, which is extended compared to homologs, functions as a means to remain bound to DNA, protecting the labile abasic site while waiting for its handoff with APE1. This was found through truncation of the N-terminal region (residues 1-96), which revealed that deletion of 55, 75, or 80 residues from the N-terminus resulted in a four to fivefold increase in catalytic activity. The rate-limiting step in hNTHL1's reaction is the release of the free 3’ aldehyde <ref>PMID:12144783</ref>. | The function of the <scene name='10/1077482/N-terminus_AlphaFold/3'>N-terminus</scene> (AlphaFold Prediction) of hNTHL1 has been a subject of study <ref>https://www.nature.com/articles/s41586-021-03819-2/</ref><ref>PMID:37933859</ref>. It is theorized that the N-terminus, which is extended compared to homologs, functions as a means to remain bound to DNA, protecting the labile abasic site while waiting for its handoff with APE1. This was found through truncation of the N-terminal region (residues 1-96), which revealed that deletion of 55, 75, or 80 residues from the N-terminus resulted in a four to fivefold increase in catalytic activity. The rate-limiting step in hNTHL1's reaction is the release of the free 3’ aldehyde <ref>PMID:12144783</ref>. | ||
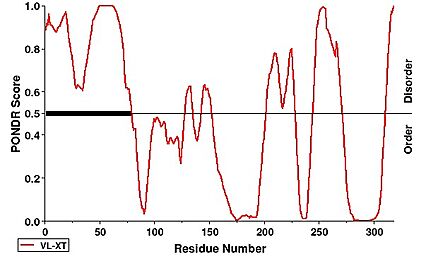
| - | Notably, the first 63 residues were not modeled | + | Notably, the first 63 residues of hNTHL1 were not modeled due to disorder. This can be observed under a PONDR prediction |
[[Image:PONDR.jpg|438 × 271px|'''PONDR Disorder Prediction''']] <ref>https://www.pondr.com/</ref>. | [[Image:PONDR.jpg|438 × 271px|'''PONDR Disorder Prediction''']] <ref>https://www.pondr.com/</ref>. | ||
| Line 35: | Line 35: | ||
== Disease == | == Disease == | ||
| - | When the base excision repair pathway is compromised, | + | When the base excision repair pathway is compromised, the ability to repair damaged DNA is significantly reduced. This turns into mutations throughout the genome, leading to the progression of cancer. '''NTHL1-Tumor Syndrome''' is a disease caused by variants affecting the gene that render the glycosylase inactive. This syndrome is diagnosed by a germline biallelic pathogenic variant through molecular genetic testing. When this is the case, one's cumulative lifetime risk of developing extracolonic cancer by age 60 is 45-78%. Oftentimes, this syndrome is characterized by an increased risk of colorectal cancer, breast cancer, and colorectal polyposis. Around 5% of colorectal cancers can be explained by germline mutations within a CRC predisposing gene. Exome sequencing has led to the identification of a homozygous nonsense mutation (c.268C>T encoding p.Q90*) in the base excision repair gene NTHL1 in three unrelated families <ref>PMID:32239880</ref><ref>PMID:25938944</ref><ref>PMID:https://www.uniprot.org/uniprot/NTH_HUMAN NTH_HUMAN</ref>. |
A functional non-mutated version of hNTHL1 can additionally play a role in cancer cell survival. In triple-negative breast cancer, the protein BCL11A is frequently overexpressed. BCL11A is a transcription factor shown to stimulate hNTHL1 activity, enhancing base excision repair (BER) and enabling cancer cells to proliferate in high levels of oxidative damage. Separately, Y-box binding protein-1 (YB-1) is overexpressed in tumor cells, and hNTHL1 can be activated through direct interaction with YB-1. This boosts its ability to process oxidized bases. This YB–1–mediated stimulation of hNTHL1 causes resistance to cisplatin, a form of chemotherapy, allowing for cancer proliferation <ref>PMID:36186110</ref><ref>PMID:18307537</ref>. | A functional non-mutated version of hNTHL1 can additionally play a role in cancer cell survival. In triple-negative breast cancer, the protein BCL11A is frequently overexpressed. BCL11A is a transcription factor shown to stimulate hNTHL1 activity, enhancing base excision repair (BER) and enabling cancer cells to proliferate in high levels of oxidative damage. Separately, Y-box binding protein-1 (YB-1) is overexpressed in tumor cells, and hNTHL1 can be activated through direct interaction with YB-1. This boosts its ability to process oxidized bases. This YB–1–mediated stimulation of hNTHL1 causes resistance to cisplatin, a form of chemotherapy, allowing for cancer proliferation <ref>PMID:36186110</ref><ref>PMID:18307537</ref>. | ||
Revision as of 00:19, 28 April 2025
Human NTHL1
| |||||||||||