User:Hayden Vissing/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
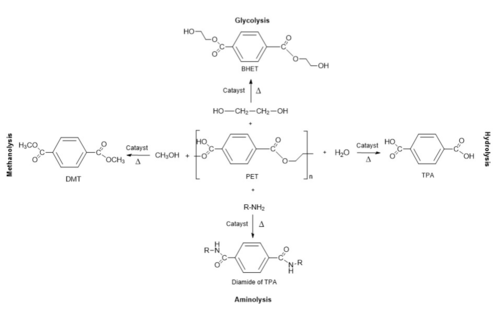
The study done by Tournier et al. (2020)<ref name="main">PMID:32269349</ref> identified a good template enzyme for creating a PET hydrolase as the LCC enzyme. This enzyme was discovered through [https://en.wikipedia.org/wiki/Metagenomics metagenomics] of a leaf-branch compost. Its target substrate is the polymer [https://en.wikipedia.org/wiki/Cutin cutin], found in the cuticle of plants. LCC breaks down Cutin to separate the monomers at the ester bond between them<ref name="breakdown">PMID:29374183</ref>. The LCC enzyme was compared to several other enzymes and was selected as it showed the best rate of PET depolymerization<ref>PMID:22194294</ref>. | The study done by Tournier et al. (2020)<ref name="main">PMID:32269349</ref> identified a good template enzyme for creating a PET hydrolase as the LCC enzyme. This enzyme was discovered through [https://en.wikipedia.org/wiki/Metagenomics metagenomics] of a leaf-branch compost. Its target substrate is the polymer [https://en.wikipedia.org/wiki/Cutin cutin], found in the cuticle of plants. LCC breaks down Cutin to separate the monomers at the ester bond between them<ref name="breakdown">PMID:29374183</ref>. The LCC enzyme was compared to several other enzymes and was selected as it showed the best rate of PET depolymerization<ref>PMID:22194294</ref>. | ||
| - | == | + | ==3-D structure== |
| + | Alpha beta folds ... | ||
| - | === | + | ===Active Site=== |
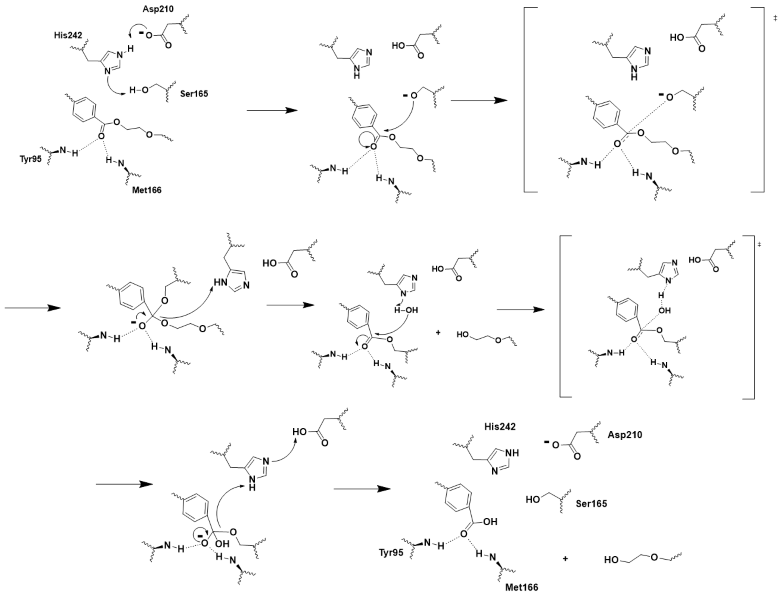
| - | LCC is a serine hydrolase | + | LCC is a [https://en.wikipedia.org/wiki/Serine_hydrolase serine hydrolase] and it functions through a <scene name='10/1076051/Wt_cat_triad/3'>catalytic triad</scene>: S165, D210, and H242. These key residues along with the <scene name='10/1075218/Oxyanion_hole/7'>oxyanion hole</scene>, M166 and Y95, and the model substrate being <scene name='10/1075218/Substrate/4'>2-HE(MHET)3</scene><ref name="substrate">A binding model of the substrate 2-HE(MHET)3 in wild-type LLC (4eb0.pdb) was constructed and refined to mimic the 3D structure illustrated in Figure 2 of reference “3”. The software Maestro (Schrödinger, Inc; version 14.2.118) was used to construct the initial binding structure, followed by energy minimization in the context of the rigid protein that had previously been processed to add/refine all hydrogen atoms. The ligand model was then used without further modification to identify and illustrate the cited active-site residues.</ref> make up the <scene name='10/1076051/Wt_active_site/2'>active site</scene><ref name="main" /> of LCC. |
| - | + | ||
| + | ===Mechanism=== | ||
| + | The chemistry involved is covalent catalysis and hydrolysis. D210 and H242 pull electron density away from S165, making it a good nucleophile and allowing it to attack the electrophilic carbonyl carbon with the oxyanion hole stabilizing the two tetrahedral transition states<ref>Han, X., Liu, W., Huang, J. W., et al. (2017). Structural insight into catalytic mechanism of PET hydrolase. Nature Communications, 8, 2106. https://doi.org/10.1038/s41467-017-02255-z.Heredia-Guerrero, J. A., Heredia, A., García-Segura, R., & Benítez, J. J. (2009). Synthesis and characterization of a plant cutin mimetic polymer. Polymer, 50(24), 5633–5637. [https://doi.org/10.1016/j.polymer.2009.10.018 DOI: 10.1016/j.polymer.2009.10.018]</ref> (Figure 3). H242 and D210 also play critical roles in general acid-base catalysis which facilitate leaving groups, step 4, and in helping water attack the electrophilic carbonyl carbon to break the covalent bond between the substrate and S165. Which as result, produces two monomers and resets the enzyme (Figure 3). | ||
[[Image:Push_mechanism.png|800px|center|thumb|Figure 3 - Push-mechanism for the cutinase reaction]] | [[Image:Push_mechanism.png|800px|center|thumb|Figure 3 - Push-mechanism for the cutinase reaction]] | ||
Revision as of 03:18, 28 April 2025
The Future of Recycling: PET Hydrolase Enzyme with Improved Efficiency and Stability
| |||||||||||
References
- ↑ Hiraga, K., Taniguchi, I., Yoshida, S., Kimura, Y., & Oda, K. (2019). Biodegradation of waste PET: A sustainable solution for dealing with plastic pollution. EMBO Reports, 20(11), e49365. https://doi.org/10.15252/embr.201949365. [Published correction appears in EMBO Reports, 21(2), e49826. DOI: 10.15252/embr.201949826
- ↑ 2.0 2.1 2.2 2.3 Jayasekara, S. K., Joni, H. D., Jayantha, B., Dissanayake, L., Mandrell, C., Sinharage, M. M. S., Molitor, R., Jayasekara, T., Sivakumar, P., & Jayakody, L. N. (2023). Trends in in-silico guided engineering of efficient polyethylene terephthalate (PET) hydrolyzing enzymes to enable bio-recycling and upcycling of PET. Computational and structural biotechnology journal, 21, 3513–3521. DOI: 10.1016/j.csbj.2023.06.004
- ↑ 3.0 3.1 Babaei, M., Jalilian, M., & Shahbaz, K. (2024). Chemical recycling of Polyethylene terephthalate: A mini-review. Journal of Environmental Chemical Engineering, 12(3), 112507. DOI: 10.1016/j.jece.2024.112507
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 4.9 Tournier V, Topham CM, Gilles A, David B, Folgoas C, Moya-Leclair E, Kamionka E, Desrousseaux ML, Texier H, Gavalda S, Cot M, Guemard E, Dalibey M, Nomme J, Cioci G, Barbe S, Chateau M, Andre I, Duquesne S, Marty A. An engineered PET depolymerase to break down and recycle plastic bottles. Nature. 2020 Apr;580(7802):216-219. doi: 10.1038/s41586-020-2149-4. Epub 2020 Apr, 8. PMID:32269349 doi:http://dx.doi.org/10.1038/s41586-020-2149-4
- ↑ Joo S, Cho IJ, Seo H, Son HF, Sagong HY, Shin TJ, Choi SY, Lee SY, Kim KJ. Structural insight into molecular mechanism of poly(ethylene terephthalate) degradation. Nat Commun. 2018 Jan 26;9(1):382. doi: 10.1038/s41467-018-02881-1. PMID:29374183 doi:http://dx.doi.org/10.1038/s41467-018-02881-1
- ↑ Sulaiman S, Yamato S, Kanaya E, Kim JJ, Koga Y, Takano K, Kanaya S. Isolation of a novel cutinase homolog with polyethylene terephthalate-degrading activity from leaf-branch compost by using a metagenomic approach. Appl Environ Microbiol. 2012 Mar;78(5):1556-62. doi: 10.1128/AEM.06725-11. Epub, 2011 Dec 22. PMID:22194294 doi:http://dx.doi.org/10.1128/AEM.06725-11
- ↑ A binding model of the substrate 2-HE(MHET)3 in wild-type LLC (4eb0.pdb) was constructed and refined to mimic the 3D structure illustrated in Figure 2 of reference “3”. The software Maestro (Schrödinger, Inc; version 14.2.118) was used to construct the initial binding structure, followed by energy minimization in the context of the rigid protein that had previously been processed to add/refine all hydrogen atoms. The ligand model was then used without further modification to identify and illustrate the cited active-site residues.
- ↑ Han, X., Liu, W., Huang, J. W., et al. (2017). Structural insight into catalytic mechanism of PET hydrolase. Nature Communications, 8, 2106. https://doi.org/10.1038/s41467-017-02255-z.Heredia-Guerrero, J. A., Heredia, A., García-Segura, R., & Benítez, J. J. (2009). Synthesis and characterization of a plant cutin mimetic polymer. Polymer, 50(24), 5633–5637. DOI: 10.1016/j.polymer.2009.10.018
Additional Literature and Resources
- Roth C, Wei R, Oeser T, Then J, Follner C, Zimmermann W, Strater N. Structural and functional studies on a thermostable polyethylene terephthalate degrading hydrolase from Thermobifida fusca. Appl Microbiol Biotechnol. 2014 Apr 13. PMID:24728714 doi:http://dx.doi.org/10.1007/s00253-014-5672-0
Student Contributors
- David Bogle
- Justin Chavez
- Hayden Vissing