We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Matthew Chien/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
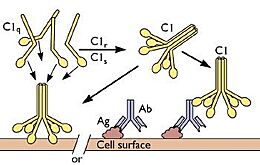
C1q plays a major role in the innate part of the immune system. It is the first complement protein to be activated in the classical complement pathway. This pathway of complement activation is dependent on the detection of antibody-antigen complexes, which creates a cascade of complement protein activation to ultimately promote inflammation, enhance the performance of various immune cells, and attack the pathogen's cell membrane. This acts as an irreplicable aspect of the innate immune system and is one of the body's primary defense against invaders. | C1q plays a major role in the innate part of the immune system. It is the first complement protein to be activated in the classical complement pathway. This pathway of complement activation is dependent on the detection of antibody-antigen complexes, which creates a cascade of complement protein activation to ultimately promote inflammation, enhance the performance of various immune cells, and attack the pathogen's cell membrane. This acts as an irreplicable aspect of the innate immune system and is one of the body's primary defense against invaders. | ||
| - | C1q is also known to be a kind of opsonin, which is a protein that tags a foreign cell to be engulfed and destroyed by phagocytes, such as neutrophils and macrophages. This process is a very important aspect of the innate immune system to eliminate foreign bacteria and viruses to keep the host healthy. It has been shown that both neutrophils and macrophages posses receptors capable of binding the globular head and CLR of C1q, named gC1q and cC1q respectively, emphasizing their crucial role for phagocytes to identify and eliminate foreign pathogens. Immature dendritic cells have also been shown to contain a large amounts of gC1q and cC1q. This acts as an important bridge between the innate and adaptive immune systems; dendritic cells can survey certain areas based on the amount of present C1q in order to activate and begin digesting antigens to present to T-cells, which is a crucial step in adaptive immunity activation. | + | C1q is also known to be a kind of opsonin, which is a protein that tags a foreign cell to be engulfed and destroyed by phagocytes, such as neutrophils and macrophages. This process is a very important aspect of the innate immune system to eliminate foreign bacteria and viruses to keep the host healthy. It has been shown that both neutrophils and macrophages posses receptors capable of binding the globular head and CLR of C1q, named gC1q and cC1q respectively, emphasizing their crucial role for phagocytes to identify and eliminate foreign pathogens. Immature dendritic cells have also been shown to contain a large amounts of gC1q and cC1q. This acts as an important bridge between the innate and adaptive immune systems; dendritic cells can survey certain areas based on the amount of present C1q in order to activate and begin digesting antigens to present to T-cells, which is a crucial step in adaptive immunity activation. <ref>PMID:9407113</ref><ref>PMID:28104818</ref> |
=== Adaptive Immunity === | === Adaptive Immunity === | ||
As iterated above, C1q is important in the activation phase of the adaptive immune response. In addition, C1q plays a major role in the solubilization of certain immune complexes, mainly the antibody-antigen complex. One of the main roles of C1q is to bind to these complexes at the Fc region of Immunoglobulin (Ig) G and IgM when bonded to an antigen. This activates the classical cascade of the complement system but also prevents the immune complex from becoming insoluble, which could lead to further unnecessary inflammation as they can no longer be detected by phagocytes for destruction. C1q, especially the globular head region, is known to be profoundly <scene name='10/1078778/Surface_Potential/1'>positively charged</scene> and is a key component in the prevention of preformed immune precipitation, along with other components of the classical complement pathway. | As iterated above, C1q is important in the activation phase of the adaptive immune response. In addition, C1q plays a major role in the solubilization of certain immune complexes, mainly the antibody-antigen complex. One of the main roles of C1q is to bind to these complexes at the Fc region of Immunoglobulin (Ig) G and IgM when bonded to an antigen. This activates the classical cascade of the complement system but also prevents the immune complex from becoming insoluble, which could lead to further unnecessary inflammation as they can no longer be detected by phagocytes for destruction. C1q, especially the globular head region, is known to be profoundly <scene name='10/1078778/Surface_Potential/1'>positively charged</scene> and is a key component in the prevention of preformed immune precipitation, along with other components of the classical complement pathway. | ||
| - | C1q has been shown to partially facilitate the function of T-cells. The receptors gC1q and cC1q are both present on T-cells and serve as both activators and inhibitors. In the presence of immune complexes, C1q has shown to activate T-cells, acting as a bridge between the innate and adaptive immune system. In terms of inhibition, C1q is observed to suppress the activity of T-cells in certain environments to regulate the immune system and prevent autoimmune disease. C1q is known to bind to various phospholipids, namely phosphatidylserine, which is largely present on the surface of apoptotic cells. This promotes opsonization by complement proteins such as C1q which leads to macrophage activation while also inhibiting T-cells to interact with the apoptotic cells. Over-activation of T-cells can lead to unnecessary harm within the body when their presence is not necessary and phagocytes are favored. | + | C1q has been shown to partially facilitate the function of T-cells. The receptors gC1q and cC1q are both present on T-cells and serve as both activators and inhibitors. In the presence of immune complexes, C1q has shown to activate T-cells, acting as a bridge between the innate and adaptive immune system. In terms of inhibition, C1q is observed to suppress the activity of T-cells in certain environments to regulate the immune system and prevent autoimmune disease. C1q is known to bind to various phospholipids, namely phosphatidylserine, which is largely present on the surface of apoptotic cells. This promotes opsonization by complement proteins such as C1q which leads to macrophage activation while also inhibiting T-cells to interact with the apoptotic cells. Over-activation of T-cells can lead to unnecessary harm within the body when their presence is not necessary and phagocytes are favored. <ref>PMID:15982298</ref><ref>PMID:20010915</ref> |
== Active Site == | == Active Site == | ||
=== Globular Head === | === Globular Head === | ||
| - | C1q is observed to have many active sites depending on what it is interacting with, with most of these active sites being located on the globular head regions. The most researched interaction concerning C1q active site is its interaction with the antibody-antigen complex. C1q largely depends on ionic residues to create salt bridges and ionic bonds with the Cγ2 domain of IgG or the Cμ3 domain of IgM. The Cγ2 of IgG is located on the Fc region, which becomes aggregated upon complexing with antigens, allowing for increased affinity to C1q. Residues Glu198 -X- Asp200 -X- Lys202 on the A chain of the C1q globular head have been shown to be a consistent active site motif on C1q. This region forms an antiparallel ß-sheet, promoting stability. Another active site has been observed on the B chain of the globular head region (ghB) at residues 114-129. The principle residues involved in binding are the Arg114 and Arg129. These residues interact with negatively charged residues on IgG, mostly Asp and Glu, such as residues Glu195 and Glu287. A common motif seen on IgG that these active sites interact with is Glu - X - Lys - X - Lys, located at residues 318, 320, and 322 respectively. This is a common motif seen on many proteins associated with the immune system, which enforces the observation that C1q can interact with many different proteins in many different contexts. One study showed that an arginine or lysine is required at positions 320 and 322 and a glutamic acid or threonine is required at position 318 in order for C1q to successfully bind. This suggests that a hydrogen bond at position 318 and an ionic interaction at positions 320 and 322 are required for C1q to bind to IgG and other epitopes. | + | C1q is observed to have many active sites depending on what it is interacting with, with most of these active sites being located on the globular head regions. The most researched interaction concerning C1q active site is its interaction with the antibody-antigen complex. C1q largely depends on ionic residues to create salt bridges and ionic bonds with the Cγ2 domain of IgG or the Cμ3 domain of IgM. The Cγ2 of IgG is located on the Fc region, which becomes aggregated upon complexing with antigens, allowing for increased affinity to C1q. Residues Glu198 -X- Asp200 -X- Lys202 on the A chain of the C1q globular head have been shown to be a consistent active site motif on C1q. This region forms an antiparallel ß-sheet, promoting stability. Another active site has been observed on the B chain of the globular head region (ghB) at residues 114-129. The principle residues involved in binding are the Arg114 and Arg129. These residues interact with negatively charged residues on IgG, mostly Asp and Glu, such as residues Glu195 and Glu287. A common motif seen on IgG that these active sites interact with is Glu - X - Lys - X - Lys, located at residues 318, 320, and 322 respectively. This is a common motif seen on many proteins associated with the immune system, which enforces the observation that C1q can interact with many different proteins in many different contexts. One study showed that an arginine or lysine is required at positions 320 and 322 and a glutamic acid or threonine is required at position 318 in order for C1q to successfully bind. This suggests that a hydrogen bond at position 318 and an ionic interaction at positions 320 and 322 are required for C1q to bind to IgG and other epitopes. <ref>PMID:8486696</ref><ref>PMID:3258649</ref> |
=== Collagen-Like Region === | === Collagen-Like Region === | ||
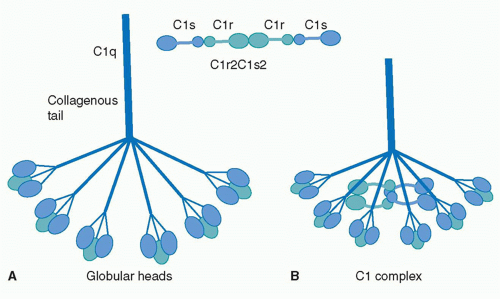
| - | The collagen-like region (CLR) is the location of a binding site associated with a variety of non-complement related interactions, such as the binding of phagocytes to C1q as mentioned above. However, one of the most important functions of the CLR of C1q is the activation through protease of the remainder of C1; C1r and C1s. Two equivalences of C1r and C1s are bonded C1s - C1r - C1r - C1s to comprise a tetramer that, when cleaved after the bonding of C1q to an epitope, activates the classical pathway of complement activity, promoting inflammation and phagocytosis. The active site on the CLR of C1q is proposed to be lysine residues located at position 61 on the B chain and position 58 on the A chain. These basic residues create salt bridges with associated residues on C1r, generally a glutamic acid or aspartic acid. These residues are coordinated with the Ca2+ ion present in C1q through single carboxyl oxygens, which is available for mediating through an electrostatic bond with a basic residue. Through activation, the C1r - C1r bond is broken, which induces conformational changes in C1r that subsequently cleaves C1s. | + | The collagen-like region (CLR) is the location of a binding site associated with a variety of non-complement related interactions, such as the binding of phagocytes to C1q as mentioned above. However, one of the most important functions of the CLR of C1q is the activation through protease of the remainder of C1; C1r and C1s. Two equivalences of C1r and C1s are bonded C1s - C1r - C1r - C1s to comprise a tetramer that, when cleaved after the bonding of C1q to an epitope, activates the classical pathway of complement activity, promoting inflammation and phagocytosis. The active site on the CLR of C1q is proposed to be lysine residues located at position 61 on the B chain and position 58 on the A chain. These basic residues create salt bridges with associated residues on C1r, generally a glutamic acid or aspartic acid. These residues are coordinated with the Ca2+ ion present in C1q through single carboxyl oxygens, which is available for mediating through an electrostatic bond with a basic residue. Through activation, the C1r - C1r bond is broken, which induces conformational changes in C1r that subsequently cleaves C1s. <ref>PMID:29311313</ref><ref>PMID:23922389</ref><ref>PMID:1939090</ref> |
== Disease == | == Disease == | ||
Revision as of 13:08, 28 April 2025
| |||||||||||
References
- ↑ Reid KBM. Complement Component C1q: Historical Perspective of a Functionally Versatile, and Structurally Unusual, Serum Protein. Front Immunol. 2018 Apr 10;9:764. PMID:29692784 doi:10.3389/fimmu.2018.00764
- ↑ Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. The complement system and innate immunity. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27100/
- ↑ Kishore U, Ghai R, Greenhough TJ, Shrive AK, Bonifati DM, Gadjeva MG, Waters P, Kojouharova MS, Chakraborty T, Agrawal A. Structural and functional anatomy of the globular domain of complement protein C1q. Immunol Lett. 2004 Sep;95(2):113-28. PMID:15388251 doi:10.1016/j.imlet.2004.06.015
- ↑ Kaul M, Loos M. Dissection of C1q capability of interacting with IgG. Time-dependent formation of a tight and only partly reversible association. J Biol Chem. 1997 Dec 26;272(52):33234-44. PMID:9407113 doi:10.1074/jbc.272.52.33234
- ↑ Mortensen SA, Sander B, Jensen RK, Pedersen JS, Golas MM, Jensenius JC, Hansen AG, Thiel S, Andersen GR. Structure and activation of C1, the complex initiating the classical pathway of the complement cascade. Proc Natl Acad Sci U S A. 2017 Jan 31;114(5):986-991. PMID:28104818 doi:10.1073/pnas.1616998114
- ↑ Sontheimer RD, Racila E, Racila DM. C1q: its functions within the innate and adaptive immune responses and its role in lupus autoimmunity. J Invest Dermatol. 2005 Jul;125(1):14-23. PMID:15982298 doi:10.1111/j.0022-202X.2005.23673.x
- ↑ Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010 Jan;20(1):34-50. PMID:20010915 doi:10.1038/cr.2009.139
- ↑ Marqués G, Antón LC, Barrio E, Sánchez A, Ruiz S, Gavilanes F, Vivanco F. Arginine residues of the globular regions of human C1q involved in the interaction with immunoglobulin G. J Biol Chem. 1993 May 15;268(14):10393-402 PMID:8486696
- ↑ Duncan AR, Winter G. The binding site for C1q on IgG. Nature. 1988 Apr 21;332(6166):738-40. PMID:3258649 doi:10.1038/332738a0
- ↑ Almitairi JOM, Venkatraman Girija U, Furze CM, Simpson-Gray X, Badakshi F, Marshall JE, Schwaeble WJ, Mitchell DA, Moody PCE, Wallis R. Structure of the C1r-C1s interaction of the C1 complex of complement activation. Proc Natl Acad Sci U S A. 2018 Jan 8. pii: 1718709115. doi:, 10.1073/pnas.1718709115. PMID:29311313 doi:http://dx.doi.org/10.1073/pnas.1718709115
- ↑ Venkatraman Girija U, Gingras AR, Marshall JE, Panchal R, Sheikh MA, Gal P, Schwaeble WJ, Mitchell DA, Moody PC, Wallis R. Structural basis of the C1q/C1s interaction and its central role in assembly of the C1 complex of complement activation. Proc Natl Acad Sci U S A. 2013 Aug 20;110(34):13916-20. doi:, 10.1073/pnas.1311113110. Epub 2013 Aug 6. PMID:23922389 doi:10.1073/pnas.1311113110
- ↑ Guan EN, Burgess WH, Robinson SL, Goodman EB, McTigue KJ, Tenner AJ. Phagocytic cell molecules that bind the collagen-like region of C1q. Involvement in the C1q-mediated enhancement of phagocytosis. J Biol Chem. 1991 Oct 25;266(30):20345-55 PMID:1939090