We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox323
From Proteopedia
(Difference between revisions)
| Line 96: | Line 96: | ||
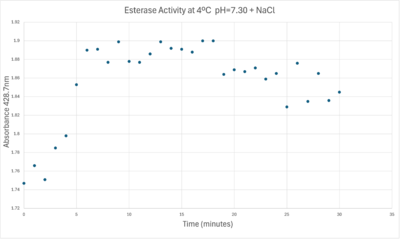
[[Image:Absorbancelongph7cold.png|400px|left|Absorbance at 405nm of a PNPA+Protein solution with pH = 7 and sodium chloride in a cold environment.]] | [[Image:Absorbancelongph7cold.png|400px|left|Absorbance at 405nm of a PNPA+Protein solution with pH = 7 and sodium chloride in a cold environment.]] | ||
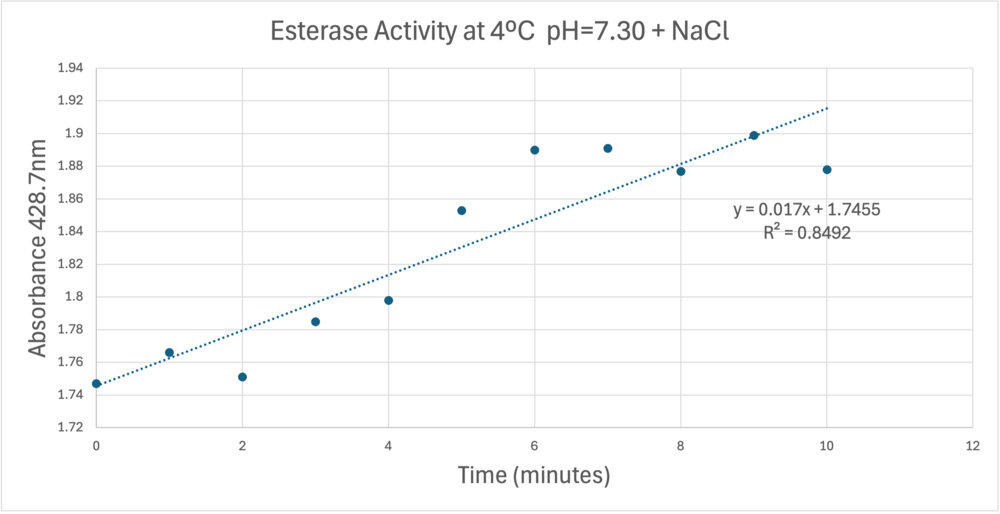
| - | [[Image:Absorbancecoldph7try.png| | + | [[Image:Absorbancecoldph7try.png|1000px|left|A trendline showing the potential relationship between absorbance and time in a PNPA+Protein solution in a solution with a pH of 7, sodium chloride, and a external temperature of 4 C.]] |
A linear equation of y=9.275x10^-7x+1x10^-4 with a coefficient of determination of 0.8492 was calculated using linear regression. | A linear equation of y=9.275x10^-7x+1x10^-4 with a coefficient of determination of 0.8492 was calculated using linear regression. | ||
| - | [[Image:Tablebeerlaw.png| | + | [[Image:Tablebeerlaw.png|1000px|left|thumb|Beer-Lambert Law sample calculation.]] |
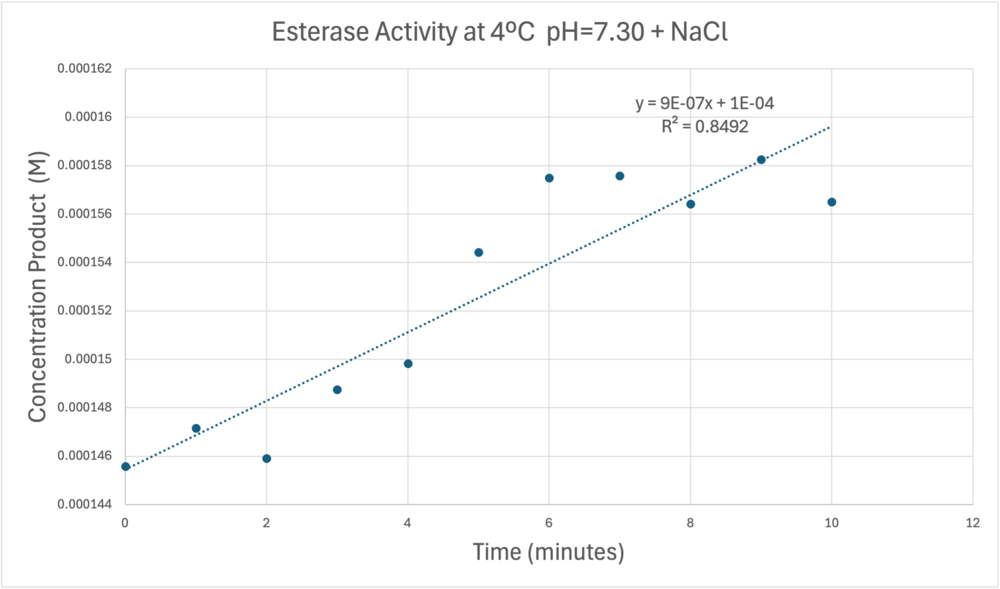
| - | [[Image:Concentrationcoldmolarabsorb12000ph7.png| | + | [[Image:Concentrationcoldmolarabsorb12000ph7.png|1000px|left|thumb|A trendline showing the potential relationship between time and product concentration from the data gathered from the PNPA+Protein solution with pH = 7, sodium chloride, and a cold environment.]] |
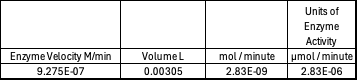
Absorbance values can be transformed to units of concentration via the Beer-Lambert law. We must accept the approximation of the Molar Extinction Coefficient for PNPA hydrolysis at 428.7nm as 12000 M^-1 cm^-1. An example calculation is supplied in the table. Graphing time versus concentration and determining the slope of the line yields the enzyme's velocity in M/min. 1mg/mL of PNPA is saturating conditions which implies the Vmax is also the slope. The reaction volume total times Vmax yields Units of Enzyme Activity. This value can be used as a relative comparison tool for enzyme performance in given conditions. | Absorbance values can be transformed to units of concentration via the Beer-Lambert law. We must accept the approximation of the Molar Extinction Coefficient for PNPA hydrolysis at 428.7nm as 12000 M^-1 cm^-1. An example calculation is supplied in the table. Graphing time versus concentration and determining the slope of the line yields the enzyme's velocity in M/min. 1mg/mL of PNPA is saturating conditions which implies the Vmax is also the slope. The reaction volume total times Vmax yields Units of Enzyme Activity. This value can be used as a relative comparison tool for enzyme performance in given conditions. | ||
| - | [[Image:Iceenzymeefficiencyscinotation.png| | + | [[Image:Iceenzymeefficiencyscinotation.png|1000px|left|thumb|Enzyme Activity at neutral pH at cold temperature with Chloride cofactor.]] |
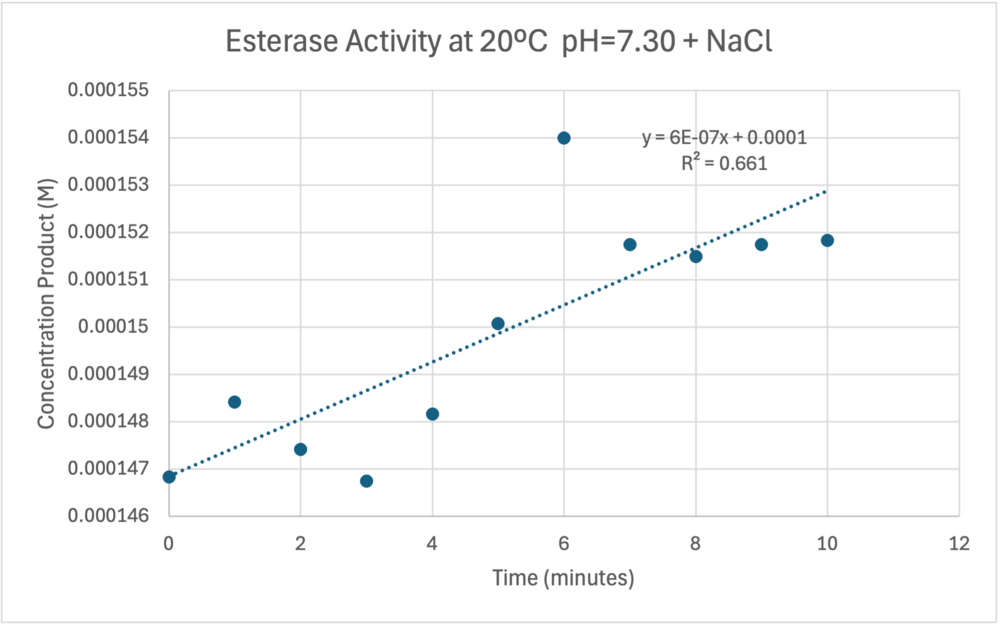
The cold 4 degrees C reaction was changed to room temperature 20 degrees C. All other conditions remained constant to evaluate the effect of temperature on enzyme. Temperature increase negatively affects enzyme performance. Considering the cold loving nature of Chitinophaga pinensis, the enzyme being more active at a lower temperature is a reasonable conclusion. | The cold 4 degrees C reaction was changed to room temperature 20 degrees C. All other conditions remained constant to evaluate the effect of temperature on enzyme. Temperature increase negatively affects enzyme performance. Considering the cold loving nature of Chitinophaga pinensis, the enzyme being more active at a lower temperature is a reasonable conclusion. | ||
| - | [[Image:Roomtempconcentrationtime.png| | + | [[Image:Roomtempconcentrationtime.png|1000px|right|thumb|Enzyme Activity at neutral pH at room temperature with Chloride cofactor.]] |
| - | [[Image:Sigfigroomtempenzymeactivity.png| | + | [[Image:Sigfigroomtempenzymeactivity.png|1000px|right|thumb|Enzyme Activity at neutral pH at room temperature with Chloride cofactor.]] |
| - | [[Image:Enzyme units percentage increase.png|1000px|thumb|4 degrees C yields 42.2% increase in Units of Enzyme Activity цmol/minute.]] | + | [[Image:Enzyme units percentage increase.png|1000px|left|thumb|4 degrees C yields 42.2% increase in Units of Enzyme Activity цmol/minute.]] |
=== Conclusion === | === Conclusion === | ||
Revision as of 16:04, 28 April 2025
Structural Analysis and Proposed Functionality of 4Q7Q
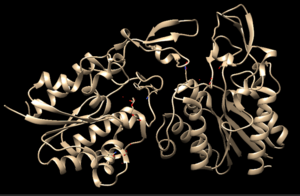
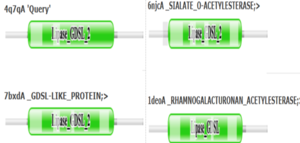
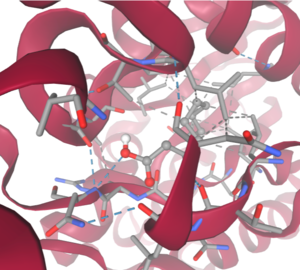
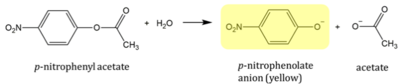
4Q7Q is a homodimeric protein complex that originates from the bacterial species Chitinophaga Pinensis and has a mass of 58.5 kDa. It is a member of the SGNH Hydrolase Superfamily with structural and sequential similarities to esterases and lipases. Current evidence suggests it causes the hydrolysis of esters and/or acetyl groups on lipids/lipid-like molecules via a catalytic triad-like active site.
| |||||||||||
References
- ↑ 1.0 1.1 4Q7Q. Protein Database, 2014. https://www.rcsb.org/structure/4Q7Q
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Nadzirin, N.; Gardiner, E.; Willett, P.; Artymiuk, P. J.; Firdaus-Raih, M. 2012. SPRITE and ASSAM: web servers for side chain 3D-motif searching in protein structures. Nucleic Acids Res., 40(Web Server Issue), W380-6.
- ↑ 3.0 3.1 Rio, T. G. D.; et al. Complete genome sequence of Chitinophaga pinensis type strain (UQM 2034). Stand. Genomic. Sci., 2010, 2(1), 87-95. https://pmc.ncbi.nlm.nih.gov/articles/PMC3035255/
- ↑ 4.0 4.1 4.2 SGNH hydrolase superfamily. InterPro, 2017. https://www.ebi.ac.uk/interpro/entry/InterPro/IPR036514/
- ↑ 5.0 5.1 5.2 Rio, T. G. D.; et al. Complete genome sequence of Chitinophaga pinensis type strain (UQM 2034). Stand. Genomic. Sci., 2010, 2(1), 87-95. https://pmc.ncbi.nlm.nih.gov/articles/PMC3035255/
- ↑ 6.0 6.1 6.2 6.3 Molgaard, A.; Kauppinen, S.; Larsen, S. Rhamnogalacturonan acetylesterase elucidates the structure and function of a new family of hydrolases. Struct., 2000, 8(4), 373-383. https://www.sciencedirect.com/science/article/pii/S0969212600001180?via%3Dihub
- ↑ UCSF Chimera--a visualization system for exploratory research and analysis. Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. J Comput Chem. 2004 Oct;25(13):1605-12.
- ↑ Akoh, C. C.; Lee, G.; Liaw, Y.; Huang, T.; Shaw, J. GDSL family of serine esterases/lipases. Prog. Lipid Res., 2004, 43(6), 534-552. https://pubmed.ncbi.nlm.nih.gov/15522763/
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 Holm L, Laiho A, Toronen P, Salgado M (2023) DALI shines a light on remote homologs: one hundred discoveries. Protein Science 23, e4519
- ↑ 10.0 10.1 10.2 10.3 Bugnon M, Röhrig UF, Goullieux M, Perez MAS, Daina A, Michielin O, Zoete V. SwissDock 2024: major enhancements for small-molecule docking with Attracting Cavities and AutoDock Vina. Nucleic Acids Res. 2024, 52 (W1), W324-W332. DOI: 10.1093/nar/gkae300.
- ↑ 11.0 11.1 11.2 11.3 Grosdidier A, Zoete V, Michielin O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39 (Web Server issue), W270-W277. DOI: 10.1093/nar/gkr366
- ↑ 12.0 12.1 12.2 12.3 Eberhardt J, Santos-Martins D, Tillack AF, Forli S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model., 2021, 61 (8), 3891–3898, DOI: 10.1021/acs.jcim.1c00203
- ↑ 13.0 13.1 13.2 13.3 Trott O, Olson AJ. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem., 2010, 31 (2), 455–461, DOI: 10.1002/jcc.21334
- ↑ Miesfeld, R. L.; McEvoy, M. M. Biochemistry, 2nd ed.; W. W. Norton & Company, 2021
- ↑ Xia, L.; Qian, M.; Cheng, F.; Wang, Y.; Han, J.; Xu, Y.; Zhang, K.; Tian, J.; Jin, Y. The effect of lactic acid bacteria on lipid metabolism and flavor of fermented sausages. Food Biosci., 2023, 56, 103172.
- ↑ 16.0 16.1 Bornscheuer, U. T. Microbial carboxyl esterases: classification, properties and application in biocatalysis. FEMS Microbiol. Rev., 2002, 26(1), 73-81. https://doi.org/10.1111/j.1574-6976.2002.tb00599.x
- ↑ Schober, I.; Koblitz, J.; Carbasse, J. S.; Ebeling, C.; Schmidt, M. L.; Podstawka, A.; Gupta, R.; Ilangovan, V.; Chamanara, J.; Overmann, J.; Reimer, L. C. BacDive in 2025: the core database for prokaryotic strain data. Nucleic Acids Res., 2024, 53(D1), D748-D756, https://doi.org/10.1093/nar/gkae959
- ↑ 18.0 18.1 Protein Activity Assays Student Module. BASIL. https://docs.google.com/document/d/1G-H0tskmSVRhC0qsDau-7-EzUcUYN8kBUHFZyBStYcs/edit?tab=t.0 (accessed 04/28/25).
- ↑ Kademi, A.; Ait-Abdelkader, N.; Fakhreddine, L.; Baratti, J. Purification and characterization of a thermostable esterase from the moderate thermophile Bacillus circulans. Appl. Microbiol. Biotechnol., 2000, 54(1), 173-179, https://doi.org/10.1007/s002530000353