Sandbox Reserved 1846
From Proteopedia
(Difference between revisions)
| Line 30: | Line 30: | ||
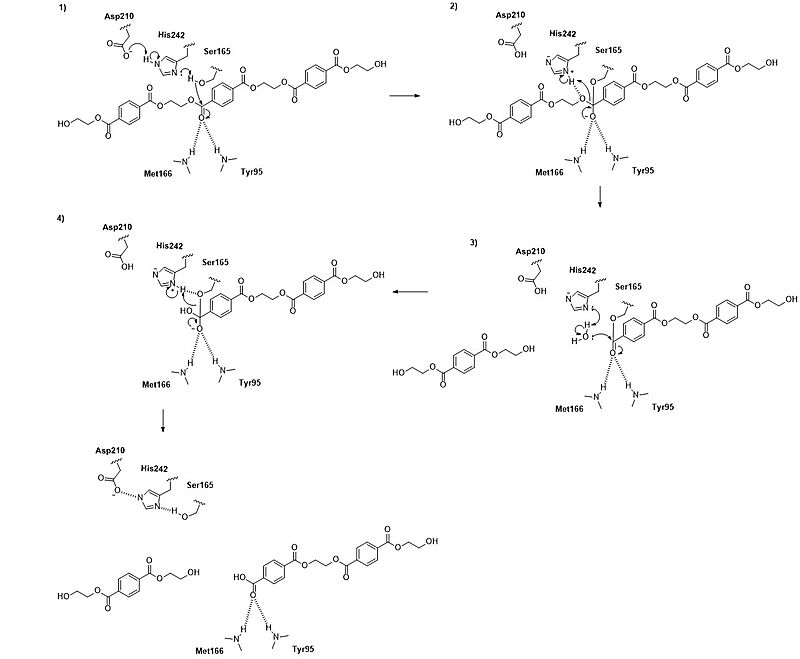
The <scene name='10/1075246/4eb0_with_colored_ligand_stick/3'>substrate-binding site</scene> of LCC is a long, mainly hydrophobic groove that accommodates PET chains. This groove includes <scene name='10/1075246/4eb0_ligand_subsites/1'>three subsites</scene> —designated −2, −1, and +1—that interact with specific PET units near the scissile ester bond. Hydrophobic residues such as <scene name='10/1075248/4eb0_with_colored_ligand_stick/2'>F125, V212, M166, and F243</scene> line the groove and facilitate substrate binding by interacting with the [https://en.wikipedia.org/wiki/Aromatic_compound aromatic] rings of the PET molecule. These interactions help align the substrate in the correct position for catalysis. | The <scene name='10/1075246/4eb0_with_colored_ligand_stick/3'>substrate-binding site</scene> of LCC is a long, mainly hydrophobic groove that accommodates PET chains. This groove includes <scene name='10/1075246/4eb0_ligand_subsites/1'>three subsites</scene> —designated −2, −1, and +1—that interact with specific PET units near the scissile ester bond. Hydrophobic residues such as <scene name='10/1075248/4eb0_with_colored_ligand_stick/2'>F125, V212, M166, and F243</scene> line the groove and facilitate substrate binding by interacting with the [https://en.wikipedia.org/wiki/Aromatic_compound aromatic] rings of the PET molecule. These interactions help align the substrate in the correct position for catalysis. | ||
| - | The <scene name='10/1075248/4eb0_surface_w_stick_ligand/1'>molecular surface view of the enzyme-ligand interaction</scene> shows the overall shape and depth of the binding groove. | + | The <scene name='10/1075248/4eb0_surface_w_stick_ligand/1'>molecular surface view of the enzyme-ligand interaction</scene> shows the overall shape and depth of the binding groove. In the <scene name='10/1075246/4eb0_hydrophobicity_ligand/3'>cartoon representation of the enzyme-ligand interaction</scene>, the enzyme is shown as a ribbon with hydrophobic residues colored pink, to show how the PET chain fits snugly into the groove. |
== Mutation Sites of Interest == | == Mutation Sites of Interest == | ||
Revision as of 22:09, 28 April 2025
Leaf Branch Compost Cutinase
| |||||||||||
References
A binding model of the substrate 2-HE(MHET)3 in wild-type LLC (4eb0.pdb) was constructed and refined to mimic the 3D structure illustrated in Figure 2 of reference [1]. The software Maestro (Schrödinger, Inc; version 14.2.118) was used to construct the initial binding structure, followed by energy minimization in the context of the rigid protein that had previously been processed to add/refine all hydrogen atoms. The ligand model was then used without further modification to identify and illustrate the cited active-site residues.- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Tournier V, Topham CM, Gilles A, David B, Folgoas C, Moya-Leclair E, Kamionka E, Desrousseaux ML, Texier H, Gavalda S, Cot M, Guemard E, Dalibey M, Nomme J, Cioci G, Barbe S, Chateau M, Andre I, Duquesne S, Marty A. An engineered PET depolymerase to break down and recycle plastic bottles. Nature. 2020 Apr;580(7802):216-219. doi: 10.1038/s41586-020-2149-4. Epub 2020 Apr, 8. PMID:32269349 doi:http://dx.doi.org/10.1038/s41586-020-2149-4
- ↑ 2.0 2.1 2.2 2.3 2.4 Sui B, Wang T, Fang J, Hou Z, Shu T, Lu Z, Liu F, Zhu Y. Recent advances in the biodegradation of polyethylene terephthalate with cutinase-like enzymes. Front Microbiol. 2023 Oct 2;14:1265139. PMID:37849919 doi:10.3389/fmicb.2023.1265139
- ↑ Lichtenthaler HK. The stress concept in plants: an introduction. Ann N Y Acad Sci. 1998 Jun 30;851:187-98. PMID:9668620 doi:10.1111/j.1749-6632.1998.tb08993.x
- ↑ Ueda H, Tabata J, Seshime Y, Masaki K, Sameshima-Yamashita Y, Kitamoto H. Cutinase-like biodegradable plastic-degrading enzymes from phylloplane yeasts have cutinase activity. Biosci Biotechnol Biochem. 2021 Jul 23;85(8):1890-1898. PMID:34160605 doi:10.1093/bbb/zbab113
- ↑ Kolattukudy PE. Biopolyester membranes of plants: cutin and suberin. Science. 1980 May 30;208(4447):990-1000. PMID:17779010 doi:10.1126/science.208.4447.990
- ↑ 6.0 6.1 6.2 6.3 6.4 Khairul Anuar NFS, Huyop F, Ur-Rehman G, Abdullah F, Normi YM, Sabullah MK, Abdul Wahab R. An Overview into Polyethylene Terephthalate (PET) Hydrolases and Efforts in Tailoring Enzymes for Improved Plastic Degradation. Int J Mol Sci. 2022 Oct 20;23(20):12644. PMID:36293501 doi:10.3390/ijms232012644
- ↑ 7.0 7.1 Burgin T, Pollard BC, Knott BC, Mayes HB, Crowley MF, McGeehan JE, Beckham GT, Woodcock HL. The reaction mechanism of the Ideonella sakaiensis PETase enzyme. Commun Chem. 2024 Mar 27;7(1):65. PMID:38538850 doi:10.1038/s42004-024-01154-x
- ↑ 8.0 8.1 8.2 Zhang J, Wang H, Luo Z, Yang Z, Zhang Z, Wang P, Li M, Zhang Y, Feng Y, Lu D, Zhu Y. Computational design of highly efficient thermostable MHET hydrolases and dual enzyme system for PET recycling. Commun Biol. 2023 Nov 9;6(1):1135. PMID:37945666 doi:10.1038/s42003-023-05523-5
- ↑ Yoshida S, Hiraga K, Takehana T, Taniguchi I, Yamaji H, Maeda Y, Toyohara K, Miyamoto K, Kimura Y, Oda K. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science. 2016 Mar 11;351(6278):1196-9. doi: 10.1126/science.aad6359. PMID:26965627 doi:http://dx.doi.org/10.1126/science.aad6359
- ↑ Landrigan PJ, Stegeman JJ, Fleming LE, Allemand D, Anderson DM, Backer LC, Brucker-Davis F, Chevalier N, Corra L, Czerucka D, Bottein MD, Demeneix B, Depledge M, Deheyn DD, Dorman CJ, Fénichel P, Fisher S, Gaill F, Galgani F, Gaze WH, Giuliano L, Grandjean P, Hahn ME, Hamdoun A, Hess P, Judson B, Laborde A, McGlade J, Mu J, Mustapha A, Neira M, Noble RT, Pedrotti ML, Reddy C, Rocklöv J, Scharler UM, Shanmugam H, Taghian G, van de Water JAJM, Vezzulli L, Weihe P, Zeka A, Raps H, Rampal P. Human Health and Ocean Pollution. Ann Glob Health. 2020 Dec 3;86(1):151. PMID:33354517 doi:10.5334/aogh.2831
- ↑ Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL. Marine pollution. Plastic waste inputs from land into the ocean. Science. 2015 Feb 13;347(6223):768-71. PMID:25678662 doi:10.1126/science.1260352
- ↑ Austin HP, Allen MD, Donohoe BS, Rorrer NA, Kearns FL, Silveira RL, Pollard BC, Dominick G, Duman R, El Omari K, Mykhaylyk V, Wagner A, Michener WE, Amore A, Skaf MS, Crowley MF, Thorne AW, Johnson CW, Woodcock HL, McGeehan JE, Beckham GT. Characterization and engineering of a plastic-degrading aromatic polyesterase. Proc Natl Acad Sci U S A. 2018 Apr 17. pii: 1718804115. doi:, 10.1073/pnas.1718804115. PMID:29666242 doi:http://dx.doi.org/10.1073/pnas.1718804115
Student Contributors
Ashley Callaghan, Rebecca Hoff, & Simone McCowan