We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Alexander Grayzel/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 27: | Line 27: | ||
=== Iron Storage === | === Iron Storage === | ||
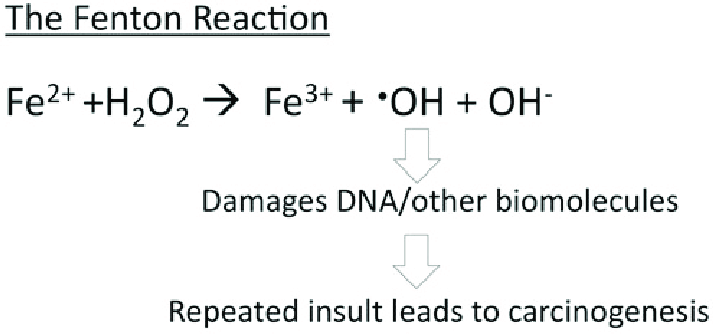
Ferritin acts as an iron delivery vehicle which brings in the Fe²⁺ form of iron to ferritin. Iron then enters ferritin through ion channels. The H-chain’s ferroxidase center oxidizes Fe²⁺ to Fe³⁺. This is then followed by nucleation and mineralization of Fe³⁺ into a ferrihydrite-like core, preventing participation in Fenton reactions that generate damaging hydroxyl radicals. Fenton reactions occur when Fe²⁺ interacts hydrogen peroxide and creates Fe³⁺, OH-, and a hydroxyl radical. This can ultimately lead to “rust”-like substances in cells which can cause DNA damage. | Ferritin acts as an iron delivery vehicle which brings in the Fe²⁺ form of iron to ferritin. Iron then enters ferritin through ion channels. The H-chain’s ferroxidase center oxidizes Fe²⁺ to Fe³⁺. This is then followed by nucleation and mineralization of Fe³⁺ into a ferrihydrite-like core, preventing participation in Fenton reactions that generate damaging hydroxyl radicals. Fenton reactions occur when Fe²⁺ interacts hydrogen peroxide and creates Fe³⁺, OH-, and a hydroxyl radical. This can ultimately lead to “rust”-like substances in cells which can cause DNA damage. | ||
| + | |||
| + | [[Image: The-Fenton-reaction-The-Fenton-reaction-involves-iron-II-Fe-2-reacting-with-H-2-O-2.png]] | ||
From a hard-soft acid-base (HSAB) perspective, this behavior is chemically intuitive. According to HSAB theory, hard acids prefer to bind with hard bases, and soft acids with soft bases. Fe³⁺ is a hard Lewis acid because it is small, highly charged, and not very polarizable. Ferritin’s iron-binding sites are rich in hard base residues such as glutamate and aspartate, which have oxygen donor atoms (hard bases). This makes the iron-glutamate/aspartate interactions highly favorable, stabilizing Fe³⁺ in the protein’s core. In contrast, Fe²⁺ is a borderline acid and is more reactive, converting it to Fe³⁺ reduces the risk of it catalyzing the harmful Fenton reactions. | From a hard-soft acid-base (HSAB) perspective, this behavior is chemically intuitive. According to HSAB theory, hard acids prefer to bind with hard bases, and soft acids with soft bases. Fe³⁺ is a hard Lewis acid because it is small, highly charged, and not very polarizable. Ferritin’s iron-binding sites are rich in hard base residues such as glutamate and aspartate, which have oxygen donor atoms (hard bases). This makes the iron-glutamate/aspartate interactions highly favorable, stabilizing Fe³⁺ in the protein’s core. In contrast, Fe²⁺ is a borderline acid and is more reactive, converting it to Fe³⁺ reduces the risk of it catalyzing the harmful Fenton reactions. | ||
| Line 33: | Line 35: | ||
Ferritin has a unique way of stabilizing the iron ions as they are transported through its protein shell. Ferritin has known chelator regions on its shell which are used to support selectivity of iron. The chelate effect occurs when a ligand has multiple donor groups for a motel ion and has an entropic effect. This means that entropy is increased favorably when the chelator is bound because instead of multiple individual ligands interacting, there is one ligand bound to the metal ion through multiple donor groups. In the case of ferritin and the context of chelation, ferritin is considered a single, polydentate ligand. This means it is a molecule with multiple donor atoms that can simultaneously bind to iron, form multiple bonds, and create a ring-like structure around the metal. | Ferritin has a unique way of stabilizing the iron ions as they are transported through its protein shell. Ferritin has known chelator regions on its shell which are used to support selectivity of iron. The chelate effect occurs when a ligand has multiple donor groups for a motel ion and has an entropic effect. This means that entropy is increased favorably when the chelator is bound because instead of multiple individual ligands interacting, there is one ligand bound to the metal ion through multiple donor groups. In the case of ferritin and the context of chelation, ferritin is considered a single, polydentate ligand. This means it is a molecule with multiple donor atoms that can simultaneously bind to iron, form multiple bonds, and create a ring-like structure around the metal. | ||
| - | |||
| - | [[Image: The-Fenton-reaction-The-Fenton-reaction-involves-iron-II-Fe-2-reacting-with-H-2-O-2.png]] | ||
=== Iron Delivery and Release === | === Iron Delivery and Release === | ||
Revision as of 21:08, 30 April 2025
Ferritin
| |||||||||||
References
Chiou, Brian, and James R Connor. “Emerging and Dynamic Biomedical Uses of Ferritin.” Pharmaceuticals (Basel, Switzerland) vol. 11,4 124. 13 Nov. 2018, doi:10.3390/ph11040124