User:Alexander Grayzel/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
<scene name='10/1078819/Ferritin_with_iron/1'>Ferritin with iron ion</scene> | <scene name='10/1078819/Ferritin_with_iron/1'>Ferritin with iron ion</scene> | ||
| - | <scene name='10/1078819/Glycerol/4'>Glycerol</scene> | ||
| - | |||
| - | <scene name='10/1078819/3-fold_channel_of_ferritin/1'>3-fold channel of ferritin</scene> | ||
| - | |||
| - | <scene name='10/1078819/4-fold_channel_of_ferritin/2'>4-fold channel of ferritin</scene> | ||
== Mechanism == | == Mechanism == | ||
=== Iron Storage === | === Iron Storage === | ||
| Line 28: | Line 23: | ||
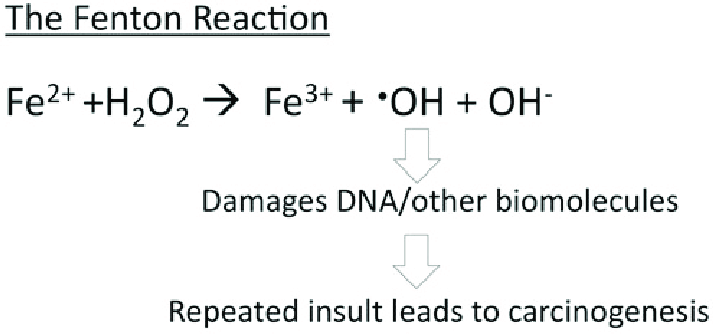
From a hard-soft acid-base (HSAB) perspective, this behavior is chemically intuitive. According to HSAB theory, hard acids prefer to bind with hard bases, and soft acids with soft bases. Fe³⁺ is a hard Lewis acid because it is small, highly charged, and not very polarizable. Ferritin’s iron-binding sites are rich in hard base residues such as glutamate and aspartate, which have oxygen donor atoms (hard bases). This makes the iron-glutamate/aspartate interactions highly favorable, stabilizing Fe³⁺ in the protein’s core. In contrast, Fe²⁺ is a borderline acid and is more reactive, converting it to Fe³⁺ reduces the risk of it catalyzing the harmful Fenton reactions. | From a hard-soft acid-base (HSAB) perspective, this behavior is chemically intuitive. According to HSAB theory, hard acids prefer to bind with hard bases, and soft acids with soft bases. Fe³⁺ is a hard Lewis acid because it is small, highly charged, and not very polarizable. Ferritin’s iron-binding sites are rich in hard base residues such as glutamate and aspartate, which have oxygen donor atoms (hard bases). This makes the iron-glutamate/aspartate interactions highly favorable, stabilizing Fe³⁺ in the protein’s core. In contrast, Fe²⁺ is a borderline acid and is more reactive, converting it to Fe³⁺ reduces the risk of it catalyzing the harmful Fenton reactions. | ||
| - | Going into further detail, Fe²⁺ ions are brought into the core through the 3-fold channel formed by the subunits of ferritin. As stated previously, the 3-fold channels are comprised primarily of aspartate and glutamate residues which makes the pore hydrophilic. This hydrophilicity allows for diffusion of water, metal cations, and hydrophilic molecules into the core. The 3-fold channel is hypothesized to be the main channel for iron entering the core. On the other hand, the 4-fold channels are responsible for diffusion of oxygen and hydrogen peroxide, not iron. It is lined with non-polar residues such as leucine and ultimately makes the channel hydrophobic. This hydrophobicity allows for diffusion of oxygen and hydrogen peroxide into and out of the ferritin core. | + | Going into further detail, Fe²⁺ ions are brought into the core through the <scene name='10/1078819/3-fold_channel_of_ferritin/1'>3-fold channel</scene> formed by the subunits of ferritin. As stated previously, the 3-fold channels are comprised primarily of aspartate and glutamate residues which makes the pore hydrophilic. This hydrophilicity allows for diffusion of water, metal cations, and hydrophilic molecules into the core. The 3-fold channel is hypothesized to be the main channel for iron entering the core. On the other hand, the <scene name='10/1078819/4-fold_channel_of_ferritin/2'>4-fold channels</scene> are responsible for diffusion of oxygen and hydrogen peroxide, not iron. It is lined with non-polar residues such as leucine and ultimately makes the channel hydrophobic. This hydrophobicity allows for diffusion of oxygen and hydrogen peroxide into and out of the ferritin core. |
Ferritin has a unique way of stabilizing the iron ions as they are transported through its protein shell. Ferritin has known chelator regions on its shell which are used to support selectivity of iron. The chelate effect occurs when a ligand has multiple donor groups for a motel ion and has an entropic effect. This means that entropy is increased favorably when the chelator is bound because instead of multiple individual ligands interacting, there is one ligand bound to the metal ion through multiple donor groups. In the case of ferritin and the context of chelation, ferritin is considered a single, polydentate ligand. This means it is a molecule with multiple donor atoms that can simultaneously bind to iron, form multiple bonds, and create a ring-like structure around the metal. | Ferritin has a unique way of stabilizing the iron ions as they are transported through its protein shell. Ferritin has known chelator regions on its shell which are used to support selectivity of iron. The chelate effect occurs when a ligand has multiple donor groups for a motel ion and has an entropic effect. This means that entropy is increased favorably when the chelator is bound because instead of multiple individual ligands interacting, there is one ligand bound to the metal ion through multiple donor groups. In the case of ferritin and the context of chelation, ferritin is considered a single, polydentate ligand. This means it is a molecule with multiple donor atoms that can simultaneously bind to iron, form multiple bonds, and create a ring-like structure around the metal. | ||
| Line 42: | Line 37: | ||
=== Diffusion Control === | === Diffusion Control === | ||
| - | Glycerol is a ligand in ferratin and plays a role in controlling diffusion of water and influencing protein stability. Specifically, glycerol dictates diffusion rates, which can allow for a stronger understanding of how diffusion affects relaxation rates of protons near ferritin. On top of this, glycerol also has the ability to morph its diffusion pores to prevent water from entering the core and causing oxidation or release water from its core for further space for iron. Additionally, glycerol has the ability to stabilize ferritin and inhibit protein aggregation during refolding. However, glycerol does not have a direct impact of iron diffusion into the core. | + | <scene name='10/1078819/Glycerol/4'>Glycerol</scene> is a ligand in ferratin and plays a role in controlling diffusion of water and influencing protein stability. Specifically, glycerol dictates diffusion rates, which can allow for a stronger understanding of how diffusion affects relaxation rates of protons near ferritin. On top of this, glycerol also has the ability to morph its diffusion pores to prevent water from entering the core and causing oxidation or release water from its core for further space for iron. Additionally, glycerol has the ability to stabilize ferritin and inhibit protein aggregation during refolding. However, glycerol does not have a direct impact of iron diffusion into the core. |
== Evolution == | == Evolution == | ||
Revision as of 21:18, 30 April 2025
Ferritin
| |||||||||||
References
Chiou, Brian, and James R Connor. “Emerging and Dynamic Biomedical Uses of Ferritin.” Pharmaceuticals (Basel, Switzerland) vol. 11,4 124. 13 Nov. 2018, doi:10.3390/ph11040124