We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Alexander Grayzel/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
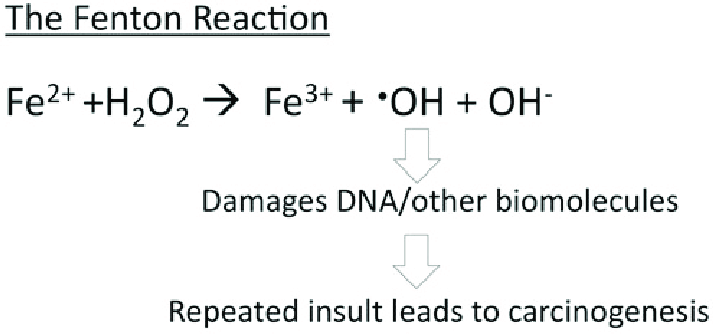
Ferritin acts as an iron delivery vehicle which brings in the Fe²⁺ form of iron to ferritin. Iron then enters ferritin through ion channels. The H-chain’s ferroxidase center oxidizes Fe²⁺ to Fe³⁺. This is then followed by nucleation and mineralization of Fe³⁺ into a ferrihydrite-like core, preventing participation in Fenton reactions that generate damaging hydroxyl radicals. Fenton reactions occur when Fe²⁺ interacts hydrogen peroxide and creates Fe³⁺, OH-, and a hydroxyl radical.<ref name="Bystrom">Bystrom, L. M., Guzman, M. L., & Rivella, S. (2014). Iron and reactive oxygen species: friends or foes of cancer cells?. Antioxidants & redox signaling, 20(12), 1917–1924. https://doi.org/10.1089/ars.2012.5014</ref> This can ultimately lead to “rust”-like substances in cells which can cause DNA damage. | Ferritin acts as an iron delivery vehicle which brings in the Fe²⁺ form of iron to ferritin. Iron then enters ferritin through ion channels. The H-chain’s ferroxidase center oxidizes Fe²⁺ to Fe³⁺. This is then followed by nucleation and mineralization of Fe³⁺ into a ferrihydrite-like core, preventing participation in Fenton reactions that generate damaging hydroxyl radicals. Fenton reactions occur when Fe²⁺ interacts hydrogen peroxide and creates Fe³⁺, OH-, and a hydroxyl radical.<ref name="Bystrom">Bystrom, L. M., Guzman, M. L., & Rivella, S. (2014). Iron and reactive oxygen species: friends or foes of cancer cells?. Antioxidants & redox signaling, 20(12), 1917–1924. https://doi.org/10.1089/ars.2012.5014</ref> This can ultimately lead to “rust”-like substances in cells which can cause DNA damage. | ||
| - | [[Image: The-Fenton-reaction-The-Fenton-reaction-involves-iron-II-Fe-2-reacting-with-H-2-O-2.png]]Figure 1. The Fenton reaction. This reaction involves iron(II) reacting with hydrogen peroxide (H2O2), making a hydroxyl radical and hydroxide ion.<ref name="Bystrom" /> | + | [[Image: The-Fenton-reaction-The-Fenton-reaction-involves-iron-II-Fe-2-reacting-with-H-2-O-2.png]]'''Figure 1.''' The Fenton reaction. This reaction involves iron(II) reacting with hydrogen peroxide (H2O2), making a hydroxyl radical and hydroxide ion.<ref name="Bystrom" /> |
From a hard-soft acid-base (HSAB) perspective, this behavior is chemically intuitive. According to HSAB theory, hard acids prefer to bind with hard bases, and soft acids with soft bases.<ref name="Lopachin">Lopachin, R. M., Gavin, T., Decaprio, A., & Barber, D. S. (2012). Application of the Hard and Soft, Acids and Bases (HSAB) theory to toxicant--target interactions. Chemical research in toxicology, 25(2), 239–251. https://doi.org/10.1021/tx2003257</ref> Fe³⁺ is a hard Lewis acid because it is small, highly charged, and not very polarizable. Ferritin’s iron-binding sites are rich in hard base residues such as glutamate and aspartate, which have oxygen donor atoms (hard bases).<ref name="Lopachin" /> This makes the iron-glutamate/aspartate interactions highly favorable, stabilizing Fe³⁺ in the protein’s core. In contrast, Fe²⁺ is a borderline acid and is more reactive, converting it to Fe³⁺ reduces the risk of it catalyzing the harmful Fenton reactions. | From a hard-soft acid-base (HSAB) perspective, this behavior is chemically intuitive. According to HSAB theory, hard acids prefer to bind with hard bases, and soft acids with soft bases.<ref name="Lopachin">Lopachin, R. M., Gavin, T., Decaprio, A., & Barber, D. S. (2012). Application of the Hard and Soft, Acids and Bases (HSAB) theory to toxicant--target interactions. Chemical research in toxicology, 25(2), 239–251. https://doi.org/10.1021/tx2003257</ref> Fe³⁺ is a hard Lewis acid because it is small, highly charged, and not very polarizable. Ferritin’s iron-binding sites are rich in hard base residues such as glutamate and aspartate, which have oxygen donor atoms (hard bases).<ref name="Lopachin" /> This makes the iron-glutamate/aspartate interactions highly favorable, stabilizing Fe³⁺ in the protein’s core. In contrast, Fe²⁺ is a borderline acid and is more reactive, converting it to Fe³⁺ reduces the risk of it catalyzing the harmful Fenton reactions. | ||
Revision as of 22:42, 30 April 2025
Ferritin
| |||||||||||
References
- ↑ Carmona, F., Palacios, Ò., Gálvez, N., Cuesta, R., Atrian, S., Capdevila, M., & Domínguez-Vera, J. M. (n.d.). Ferritin iron uptake and release in the presence of metals and metalloproteins: Chemical implications in the brain.
- ↑ Knovich, M. A.; Storey, J. A.; Coffman, L. G.; Torti, S. V. Ferritin for the Clinician. Blood Rev 2009, 23 (3), 95–104.
- ↑ Bradley, J. M.; Le Brun, N. E.; Moore, G. R. Ferritins: Furnishing Proteins with Iron. JBIC Journal of Biological Inorganic Chemistry 2016, 21 (1), 13–28.
- ↑ 4.0 4.1 Srivastava, A.K., Reutovich, A.A., Hunter, N.J. et al. Ferritin microheterogeneity, subunit composition, functional, and physiological implications. Sci Rep 13, 19862 (2023). https://doi.org/10.1038/s41598-023-46880-9
- ↑ 5.0 5.1 Levi, S., & Rovida, E. (2015). Neuroferritinopathy: From ferritin structure modification to pathogenetic mechanism. Neurobiology of disease, 81, 134–143. https://doi.org/10.1016/j.nbd.2015.02.007
- ↑ 6.0 6.1 Bystrom, L. M., Guzman, M. L., & Rivella, S. (2014). Iron and reactive oxygen species: friends or foes of cancer cells?. Antioxidants & redox signaling, 20(12), 1917–1924. https://doi.org/10.1089/ars.2012.5014
- ↑ 7.0 7.1 Lopachin, R. M., Gavin, T., Decaprio, A., & Barber, D. S. (2012). Application of the Hard and Soft, Acids and Bases (HSAB) theory to toxicant--target interactions. Chemical research in toxicology, 25(2), 239–251. https://doi.org/10.1021/tx2003257
- ↑ Takahashi, T., & Kuyucak, S. (2003). Functional properties of threefold and fourfold channels in ferritin deduced from electrostatic calculations. Biophysical journal, 84(4), 2256–2263. https://doi.org/10.1016/S0006-3495(03)75031-0
- ↑ 9.0 9.1 https://chem.libretexts.org/Courses/Duke_University/Textbook%3A_Modern_Applications_of_Chemistry_(Cox)/10%3A_Bioinorganic_Chemistry/10.04%3A_Iron_Storage-_Ferritin
- ↑ 10.0 10.1 10.2 10.3 https://www.thebloodproject.com/cases-archive/the-abcs-of-ferritin/how-does-iron-get-into-and-out-of-ferritin/#:~:text=Iron%20enters%20ferritin%20through%20pores,lysosomes%20%E2%80%93%20a%20process%20called%20ferritinophagy
- ↑ Boss, M. A., & Chris Hammel, P. (2012). The role of diffusion in ferritin-induced relaxation enhancement of protons. Journal of magnetic resonance (San Diego, Calif. : 1997), 217, 36–40. https://doi.org/10.1016/j.jmr.2012.02.005
- ↑ Kotla, N. K., Dutta, P., Parimi, S., & Das, N. K. (2022). The Role of Ferritin in Health and Disease: Recent Advances and Understandings. Metabolites, 12(7), 609. https://doi.org/10.3390/metabo12070609
- ↑ Liu, J. L., Fan, Y. G., Yang, Z. S., Wang, Z. Y., & Guo, C. (2018). Iron and Alzheimer's Disease: From Pathogenesis to Therapeutic Implications. Frontiers in neuroscience, 12, 632. https://doi.org/10.3389/fnins.2018.00632