We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Alexander Grayzel/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 27: | Line 27: | ||
Ferritin has a unique way of stabilizing the iron ions as they are transported through its protein shell. Ferritin has known chelator regions on its shell which are used to support selectivity of iron. The chelate effect occurs when a ligand has multiple donor groups for a motel ion and has an entropic effect.<ref name="LibreText" /> This means that entropy is increased favorably when the chelator is bound because instead of multiple individual ligands interacting, there is one ligand bound to the metal ion through multiple donor groups. In the case of ferritin and the context of chelation, ferritin is considered a single, polydentate ligand. This means it is a molecule with multiple donor atoms that can simultaneously bind to iron, form multiple bonds, and create a ring-like structure around the metal. | Ferritin has a unique way of stabilizing the iron ions as they are transported through its protein shell. Ferritin has known chelator regions on its shell which are used to support selectivity of iron. The chelate effect occurs when a ligand has multiple donor groups for a motel ion and has an entropic effect.<ref name="LibreText" /> This means that entropy is increased favorably when the chelator is bound because instead of multiple individual ligands interacting, there is one ligand bound to the metal ion through multiple donor groups. In the case of ferritin and the context of chelation, ferritin is considered a single, polydentate ligand. This means it is a molecule with multiple donor atoms that can simultaneously bind to iron, form multiple bonds, and create a ring-like structure around the metal. | ||
| + | |||
| + | [[Image:https://ars.els-cdn.com/content/image/1-s2.0-S0304416510000954-gr1.jpg]] | ||
| + | '''Figure 2.''' Ferritin Chelation. The left panel shows a ribbon model of the ferritin subunit, highlighting key helices (A-E) and loop L that form the protein's outer and inner shell. Iron ions (Fe) are visualized within the protein core, with the ferroxidase center located between helices A and B. The right zooms into the ferroxidase center, illustrating the coordination of two iron atoms (Fe_A and Fe_B) by conserved amino acid residues including Glu-27, Glu-62, His-65, Glu-107, and Gln-141. This center facilitates the oxidation and storage of Fe²⁺ as Fe³⁺ within ferritin.<ref>Bou-Abdallah F. (2010). The iron redox and hydrolysis chemistry of the ferritins. Biochimica et biophysica acta, 1800(8), 719–731. https://doi.org/10.1016/j.bbagen.2010.03.021</ref> | ||
=== Iron Delivery and Release === | === Iron Delivery and Release === | ||
| Line 36: | Line 39: | ||
Ultimately, ferritinophagy, mediated by NCOA4, is a tightly regulated process crucial for balancing iron storage and mobilization in response to the body’s needs. | Ultimately, ferritinophagy, mediated by NCOA4, is a tightly regulated process crucial for balancing iron storage and mobilization in response to the body’s needs. | ||
| + | |||
| + | [[Image:https://media.springernature.com/lw685/springer-static/image/art%3A10.1038%2Fs41420-023-01753-y/MediaObjects/41420_2023_1753_Fig1_HTML.png]] | ||
| + | '''Figure 3.''' Pathway of NCOA4 Receptor. Iron enters the cell via transferrin receptor 1 (TfR1) and is stored in ferritin. NCOA4 binds to the ferritin heavy chain (FTH1) and delivers it to autophagosomes by interacting with LC3. This complex is then transported to the autolysosome, where ferritin is broken down to release iron in its ferrous form.<ref>Wang, J., Wu, N., Peng, M. et al. Ferritinophagy: research advance and clinical significance in cancers. Cell Death Discov. 9, 463 (2023). https://doi.org/10.1038/s41420-023-01753-y</ref> | ||
=== Diffusion Control === | === Diffusion Control === | ||
Revision as of 23:20, 30 April 2025
Ferritin
| |||||||||||
References
- ↑ Carmona, F., Palacios, Ò., Gálvez, N., Cuesta, R., Atrian, S., Capdevila, M., & Domínguez-Vera, J. M. (n.d.). Ferritin iron uptake and release in the presence of metals and metalloproteins: Chemical implications in the brain.
- ↑ Knovich, M. A.; Storey, J. A.; Coffman, L. G.; Torti, S. V. Ferritin for the Clinician. Blood Rev 2009, 23 (3), 95–104.
- ↑ Bradley, J. M.; Le Brun, N. E.; Moore, G. R. Ferritins: Furnishing Proteins with Iron. JBIC Journal of Biological Inorganic Chemistry 2016, 21 (1), 13–28.
- ↑ 4.0 4.1 Srivastava, A.K., Reutovich, A.A., Hunter, N.J. et al. Ferritin microheterogeneity, subunit composition, functional, and physiological implications. Sci Rep 13, 19862 (2023). https://doi.org/10.1038/s41598-023-46880-9
- ↑ 5.0 5.1 Levi, S., & Rovida, E. (2015). Neuroferritinopathy: From ferritin structure modification to pathogenetic mechanism. Neurobiology of disease, 81, 134–143. https://doi.org/10.1016/j.nbd.2015.02.007
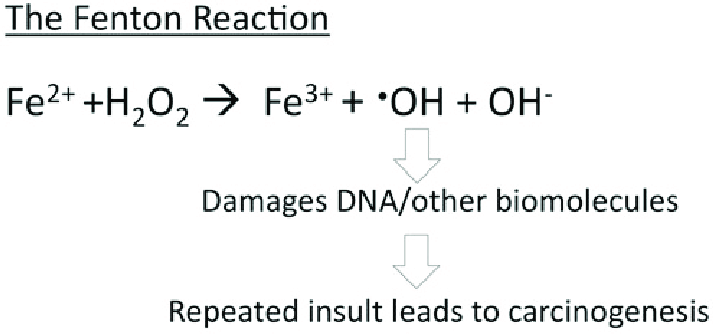
- ↑ 6.0 6.1 Bystrom, L. M., Guzman, M. L., & Rivella, S. (2014). Iron and reactive oxygen species: friends or foes of cancer cells?. Antioxidants & redox signaling, 20(12), 1917–1924. https://doi.org/10.1089/ars.2012.5014
- ↑ 7.0 7.1 Lopachin, R. M., Gavin, T., Decaprio, A., & Barber, D. S. (2012). Application of the Hard and Soft, Acids and Bases (HSAB) theory to toxicant--target interactions. Chemical research in toxicology, 25(2), 239–251. https://doi.org/10.1021/tx2003257
- ↑ Takahashi, T., & Kuyucak, S. (2003). Functional properties of threefold and fourfold channels in ferritin deduced from electrostatic calculations. Biophysical journal, 84(4), 2256–2263. https://doi.org/10.1016/S0006-3495(03)75031-0
- ↑ 9.0 9.1 https://chem.libretexts.org/Courses/Duke_University/Textbook%3A_Modern_Applications_of_Chemistry_(Cox)/10%3A_Bioinorganic_Chemistry/10.04%3A_Iron_Storage-_Ferritin
- ↑ Bou-Abdallah F. (2010). The iron redox and hydrolysis chemistry of the ferritins. Biochimica et biophysica acta, 1800(8), 719–731. https://doi.org/10.1016/j.bbagen.2010.03.021
- ↑ 11.0 11.1 11.2 11.3 https://www.thebloodproject.com/cases-archive/the-abcs-of-ferritin/how-does-iron-get-into-and-out-of-ferritin/#:~:text=Iron%20enters%20ferritin%20through%20pores,lysosomes%20%E2%80%93%20a%20process%20called%20ferritinophagy
- ↑ Wang, J., Wu, N., Peng, M. et al. Ferritinophagy: research advance and clinical significance in cancers. Cell Death Discov. 9, 463 (2023). https://doi.org/10.1038/s41420-023-01753-y
- ↑ Boss, M. A., & Chris Hammel, P. (2012). The role of diffusion in ferritin-induced relaxation enhancement of protons. Journal of magnetic resonance (San Diego, Calif. : 1997), 217, 36–40. https://doi.org/10.1016/j.jmr.2012.02.005
- ↑ Kotla, N. K., Dutta, P., Parimi, S., & Das, N. K. (2022). The Role of Ferritin in Health and Disease: Recent Advances and Understandings. Metabolites, 12(7), 609. https://doi.org/10.3390/metabo12070609
- ↑ Liu, J. L., Fan, Y. G., Yang, Z. S., Wang, Z. Y., & Guo, C. (2018). Iron and Alzheimer's Disease: From Pathogenesis to Therapeutic Implications. Frontiers in neuroscience, 12, 632. https://doi.org/10.3389/fnins.2018.00632