Thermal hysteresis protein YL-1
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
<Structure load='1ezg' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | <Structure load='1ezg' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | ||
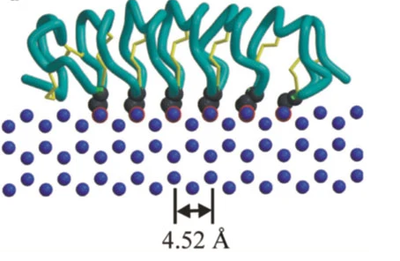
| - | [[Image:IceBind 4.png |thumb|400px| The | + | [[Image:IceBind 4.png |thumb|400px| The beta sheet roll motif lines up the amino acid chain such that each turn interacts with a unit cell of the water ice crystal]] |
Revision as of 02:44, 1 May 2025
|
Tenebrio molitor (Yellow mealworm beetle)
Contributes to protect body fluid from freezing at subzero temperatures. Lowers the freezing point of the hemolymph by about 2.5 degrees at a concentration of 1 mg/ml. Binds to nascent ice crystals and prevents further growth
Structural highlights
References
Liou YC, Tocilj A, Davies PL, Jia Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature. 2000 Jul 20;406(6793):322-4. doi: 10.1038/35018604. PMID: 10917536.