Thermal hysteresis protein YL-1
From Proteopedia
| Line 7: | Line 7: | ||
Contributes to protect body fluid from freezing at subzero temperatures. Lowers the freezing point of the hemolymph by about 2.5 degrees at a concentration of 1 mg/ml. Binds to nascent ice crystals and prevents further growth | Contributes to protect body fluid from freezing at subzero temperatures. Lowers the freezing point of the hemolymph by about 2.5 degrees at a concentration of 1 mg/ml. Binds to nascent ice crystals and prevents further growth | ||
== Structural highlights == | == Structural highlights == | ||
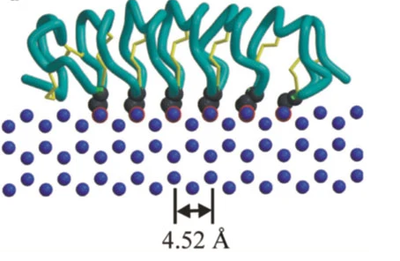
| - | <scene name='10/1080036/Ice-binding_site/1'>Ice-binding</scene> | + | The beta-sheet roll motif sets up several lines of mostly the amino acid threonine which is able to hydrogen bond to water molecules and form a <scene name='10/1080036/Ice-binding_site/1'>Ice-binding</scene> |
| - | <scene name='10/1080036/Cysteine_sulfur_bridges/1'>Cysteine sulfur bridges</scene> | + | The beta-sheet roll is held together by <scene name='10/1080036/Cysteine_sulfur_bridges/1'>Cysteine sulfur bridges</scene> which helps create the roll structure that allows this protein to position its amino acids in the manner shown. |
== References == | == References == | ||
<references/> | <references/> | ||
Liou YC, Tocilj A, Davies PL, Jia Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature. 2000 Jul 20;406(6793):322-4. doi: 10.1038/35018604. PMID: 10917536. | Liou YC, Tocilj A, Davies PL, Jia Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature. 2000 Jul 20;406(6793):322-4. doi: 10.1038/35018604. PMID: 10917536. | ||
Current revision
|
Tenebrio molitor (Yellow mealworm beetle)
Contributes to protect body fluid from freezing at subzero temperatures. Lowers the freezing point of the hemolymph by about 2.5 degrees at a concentration of 1 mg/ml. Binds to nascent ice crystals and prevents further growth
Structural highlights
The beta-sheet roll motif sets up several lines of mostly the amino acid threonine which is able to hydrogen bond to water molecules and form a The beta-sheet roll is held together by which helps create the roll structure that allows this protein to position its amino acids in the manner shown.
References
Liou YC, Tocilj A, Davies PL, Jia Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature. 2000 Jul 20;406(6793):322-4. doi: 10.1038/35018604. PMID: 10917536.