We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Alexander Grayzel/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 21: | Line 21: | ||
Ferritin has a unique way of stabilizing the iron ions as they are transported through its protein shell. Ferritin has known chelator regions on its shell which are used to support selectivity of iron. The chelate effect occurs when a ligand has multiple donor groups for a motel ion and has an entropic effect.<ref name="LibreText" /> This means that entropy is increased favorably when the chelator is bound because instead of multiple individual ligands interacting, there is one ligand bound to the metal ion through multiple donor groups. In the case of ferritin and the context of chelation, ferritin is considered a single, polydentate ligand. This means it is a molecule with multiple donor atoms that can simultaneously bind to iron, form multiple bonds, and create a ring-like structure around the metal. | Ferritin has a unique way of stabilizing the iron ions as they are transported through its protein shell. Ferritin has known chelator regions on its shell which are used to support selectivity of iron. The chelate effect occurs when a ligand has multiple donor groups for a motel ion and has an entropic effect.<ref name="LibreText" /> This means that entropy is increased favorably when the chelator is bound because instead of multiple individual ligands interacting, there is one ligand bound to the metal ion through multiple donor groups. In the case of ferritin and the context of chelation, ferritin is considered a single, polydentate ligand. This means it is a molecule with multiple donor atoms that can simultaneously bind to iron, form multiple bonds, and create a ring-like structure around the metal. | ||
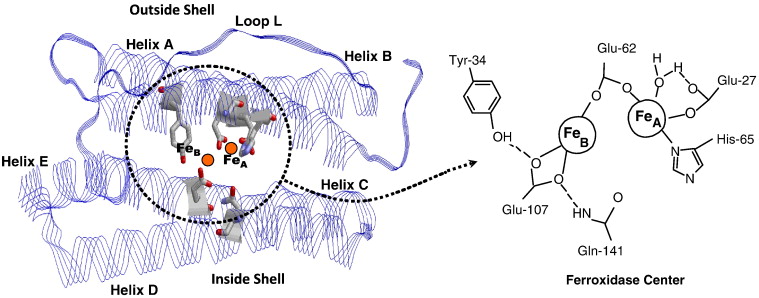
| - | [[Image: | + | [[Image: 1-s2.0-S0304416510000954-gr1.jpg]] |
| - | '''Figure | + | '''Figure 1.''' Ferritin Chelation. The left panel shows a ribbon model of the ferritin subunit, highlighting key helices (A-E) and loop L that form the protein's outer and inner shell. Iron ions (Fe) are visualized within the protein core, with the ferroxidase center located between helices A and B. The right zooms into the ferroxidase center, illustrating the coordination of two iron atoms (Fe_A and Fe_B) by conserved amino acid residues including Glu-27, Glu-62, His-65, <scene name='10/1078819/Single_ferritin_chain_with_iro/2'>Glu-107</scene>, and Gln-141. This center facilitates the oxidation and storage of Fe²⁺ as Fe³⁺ within ferritin.<ref>Bou-Abdallah F. (2010). The iron redox and hydrolysis chemistry of the ferritins. Biochimica et biophysica acta, 1800(8), 719–731. https://doi.org/10.1016/j.bbagen.2010.03.021</ref> |
The H-chain’s ferroxidase center oxidizes Fe²⁺ to Fe³⁺. This is then followed by nucleation and mineralization of Fe³⁺ into a ferrihydrite-like core, preventing participation in Fenton reactions that generate damaging hydroxyl radicals. Fenton reactions occur when Fe²⁺ interacts hydrogen peroxide and creates Fe³⁺, OH-, and a hydroxyl radical.<ref name="Bystrom">Bystrom, L. M., Guzman, M. L., & Rivella, S. (2014). Iron and reactive oxygen species: friends or foes of cancer cells?. Antioxidants & redox signaling, 20(12), 1917–1924. https://doi.org/10.1089/ars.2012.5014</ref> This can ultimately lead to “rust”-like substances in cells which can cause DNA damage. | The H-chain’s ferroxidase center oxidizes Fe²⁺ to Fe³⁺. This is then followed by nucleation and mineralization of Fe³⁺ into a ferrihydrite-like core, preventing participation in Fenton reactions that generate damaging hydroxyl radicals. Fenton reactions occur when Fe²⁺ interacts hydrogen peroxide and creates Fe³⁺, OH-, and a hydroxyl radical.<ref name="Bystrom">Bystrom, L. M., Guzman, M. L., & Rivella, S. (2014). Iron and reactive oxygen species: friends or foes of cancer cells?. Antioxidants & redox signaling, 20(12), 1917–1924. https://doi.org/10.1089/ars.2012.5014</ref> This can ultimately lead to “rust”-like substances in cells which can cause DNA damage. | ||
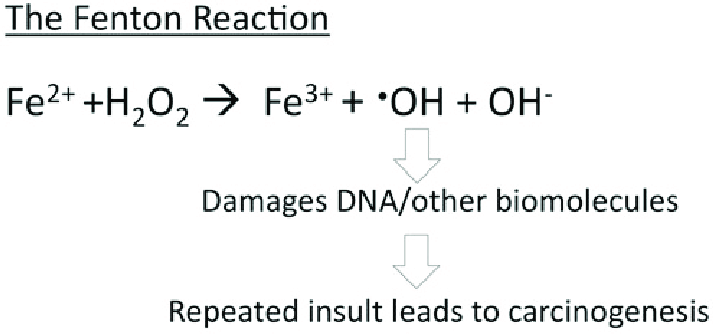
[[Image: The-Fenton-reaction-The-Fenton-reaction-involves-iron-II-Fe-2-reacting-with-H-2-O-2.png]] | [[Image: The-Fenton-reaction-The-Fenton-reaction-involves-iron-II-Fe-2-reacting-with-H-2-O-2.png]] | ||
| - | '''Figure | + | '''Figure 2.''' The Fenton reaction. This reaction involves iron(II) reacting with hydrogen peroxide (H2O2), making a hydroxyl radical and hydroxide ion.<ref name="Bystrom" /> |
Ferritin converts Fe²⁺ to Fe³⁺ inside the protein core at the ferroxidase site. This ferroxidase site gives ferritin the ability to partially be an enzyme. Fe²⁺ ions enter the ferritin protein core through channels at the 3-fold channel, as explained before. Once inside, the Fe²⁺ ions are brought to the di-iron catalytic site where they are oxidized by either dioxygen (O2) or hydrogen peroxide (H2O2).<ref name="Tosha">Tosha, T., Hasan, M. R., & Theil, E. C. (2008). The ferritin Fe2 site at the diiron catalytic center controls the reaction with O2 in the rapid mineralization pathway. Proceedings of the National Academy of Sciences of the United States of America, 105(47), 18182–18187. https://doi.org/10.1073/pnas.0805083105</ref> This oxidation process produces Fe(III)2-O products, which are precursors to the iron-rich mineral core within the ferritin. By consuming dioxygen or hydrogen peroxide, ferritin minimizes the production of harmful hydroxyl radicals that could arise from the Fenton reaction. Ultimately, the Fe(III)2-O products then from the Fe2O3H2O mineral core, where the Fe³⁺ is stored safely.<ref name="Tosha" /> | Ferritin converts Fe²⁺ to Fe³⁺ inside the protein core at the ferroxidase site. This ferroxidase site gives ferritin the ability to partially be an enzyme. Fe²⁺ ions enter the ferritin protein core through channels at the 3-fold channel, as explained before. Once inside, the Fe²⁺ ions are brought to the di-iron catalytic site where they are oxidized by either dioxygen (O2) or hydrogen peroxide (H2O2).<ref name="Tosha">Tosha, T., Hasan, M. R., & Theil, E. C. (2008). The ferritin Fe2 site at the diiron catalytic center controls the reaction with O2 in the rapid mineralization pathway. Proceedings of the National Academy of Sciences of the United States of America, 105(47), 18182–18187. https://doi.org/10.1073/pnas.0805083105</ref> This oxidation process produces Fe(III)2-O products, which are precursors to the iron-rich mineral core within the ferritin. By consuming dioxygen or hydrogen peroxide, ferritin minimizes the production of harmful hydroxyl radicals that could arise from the Fenton reaction. Ultimately, the Fe(III)2-O products then from the Fe2O3H2O mineral core, where the Fe³⁺ is stored safely.<ref name="Tosha" /> | ||
| Line 40: | Line 40: | ||
Ultimately, ferritinophagy, mediated by NCOA4, is a tightly regulated process crucial for balancing iron storage and mobilization in response to the body’s needs. | Ultimately, ferritinophagy, mediated by NCOA4, is a tightly regulated process crucial for balancing iron storage and mobilization in response to the body’s needs. | ||
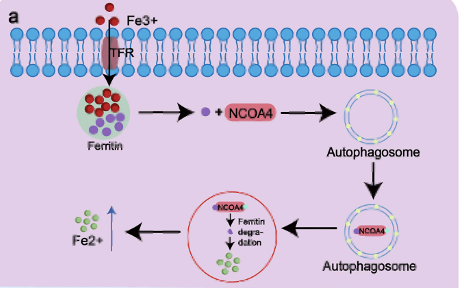
| - | [[Image: | + | [[Image: Ferritin_NCOA4_Receptor.png]] |
'''Figure 3.''' Pathway of NCOA4 Receptor. Iron enters the cell via transferrin receptor 1 (TfR1) and is stored in ferritin. NCOA4 binds to the ferritin heavy chain (FTH1) and delivers it to autophagosomes by interacting with LC3. This complex is then transported to the autolysosome, where ferritin is broken down to release iron in its ferrous form.<ref>Wang, J., Wu, N., Peng, M. et al. Ferritinophagy: research advance and clinical significance in cancers. Cell Death Discov. 9, 463 (2023). https://doi.org/10.1038/s41420-023-01753-y</ref> | '''Figure 3.''' Pathway of NCOA4 Receptor. Iron enters the cell via transferrin receptor 1 (TfR1) and is stored in ferritin. NCOA4 binds to the ferritin heavy chain (FTH1) and delivers it to autophagosomes by interacting with LC3. This complex is then transported to the autolysosome, where ferritin is broken down to release iron in its ferrous form.<ref>Wang, J., Wu, N., Peng, M. et al. Ferritinophagy: research advance and clinical significance in cancers. Cell Death Discov. 9, 463 (2023). https://doi.org/10.1038/s41420-023-01753-y</ref> | ||
Revision as of 12:12, 1 May 2025
Ferritin
| |||||||||||
References
- ↑ Carmona, F., Palacios, Ò., Gálvez, N., Cuesta, R., Atrian, S., Capdevila, M., & Domínguez-Vera, J. M. (n.d.). Ferritin iron uptake and release in the presence of metals and metalloproteins: Chemical implications in the brain.
- ↑ Knovich, M. A.; Storey, J. A.; Coffman, L. G.; Torti, S. V. Ferritin for the Clinician. Blood Rev 2009, 23 (3), 95–104.
- ↑ Bradley, J. M.; Le Brun, N. E.; Moore, G. R. Ferritins: Furnishing Proteins with Iron. JBIC Journal of Biological Inorganic Chemistry 2016, 21 (1), 13–28.
- ↑ 4.0 4.1 Srivastava, A.K., Reutovich, A.A., Hunter, N.J. et al. Ferritin microheterogeneity, subunit composition, functional, and physiological implications. Sci Rep 13, 19862 (2023). https://doi.org/10.1038/s41598-023-46880-9
- ↑ 5.0 5.1 Levi, S., & Rovida, E. (2015). Neuroferritinopathy: From ferritin structure modification to pathogenetic mechanism. Neurobiology of disease, 81, 134–143. https://doi.org/10.1016/j.nbd.2015.02.007
- ↑ Takahashi, T., & Kuyucak, S. (2003). Functional properties of threefold and fourfold channels in ferritin deduced from electrostatic calculations. Biophysical journal, 84(4), 2256–2263. https://doi.org/10.1016/S0006-3495(03)75031-0
- ↑ 7.0 7.1 https://chem.libretexts.org/Courses/Duke_University/Textbook%3A_Modern_Applications_of_Chemistry_(Cox)/10%3A_Bioinorganic_Chemistry/10.04%3A_Iron_Storage-_Ferritin
- ↑ 8.0 8.1 Lopachin, R. M., Gavin, T., Decaprio, A., & Barber, D. S. (2012). Application of the Hard and Soft, Acids and Bases (HSAB) theory to toxicant--target interactions. Chemical research in toxicology, 25(2), 239–251. https://doi.org/10.1021/tx2003257
- ↑ Bou-Abdallah F. (2010). The iron redox and hydrolysis chemistry of the ferritins. Biochimica et biophysica acta, 1800(8), 719–731. https://doi.org/10.1016/j.bbagen.2010.03.021
- ↑ 10.0 10.1 Bystrom, L. M., Guzman, M. L., & Rivella, S. (2014). Iron and reactive oxygen species: friends or foes of cancer cells?. Antioxidants & redox signaling, 20(12), 1917–1924. https://doi.org/10.1089/ars.2012.5014
- ↑ 11.0 11.1 Tosha, T., Hasan, M. R., & Theil, E. C. (2008). The ferritin Fe2 site at the diiron catalytic center controls the reaction with O2 in the rapid mineralization pathway. Proceedings of the National Academy of Sciences of the United States of America, 105(47), 18182–18187. https://doi.org/10.1073/pnas.0805083105
- ↑ 12.0 12.1 12.2 12.3 https://www.thebloodproject.com/cases-archive/the-abcs-of-ferritin/how-does-iron-get-into-and-out-of-ferritin/#:~:text=Iron%20enters%20ferritin%20through%20pores,lysosomes%20%E2%80%93%20a%20process%20called%20ferritinophagy
- ↑ Wang, J., Wu, N., Peng, M. et al. Ferritinophagy: research advance and clinical significance in cancers. Cell Death Discov. 9, 463 (2023). https://doi.org/10.1038/s41420-023-01753-y
- ↑ Boss, M. A., & Chris Hammel, P. (2012). The role of diffusion in ferritin-induced relaxation enhancement of protons. Journal of magnetic resonance (San Diego, Calif. : 1997), 217, 36–40. https://doi.org/10.1016/j.jmr.2012.02.005
- ↑ Kotla, N. K., Dutta, P., Parimi, S., & Das, N. K. (2022). The Role of Ferritin in Health and Disease: Recent Advances and Understandings. Metabolites, 12(7), 609. https://doi.org/10.3390/metabo12070609
- ↑ Liu, J. L., Fan, Y. G., Yang, Z. S., Wang, Z. Y., & Guo, C. (2018). Iron and Alzheimer's Disease: From Pathogenesis to Therapeutic Implications. Frontiers in neuroscience, 12, 632. https://doi.org/10.3389/fnins.2018.00632