We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Vinícius M. Neto/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
== Introduction == | == Introduction == | ||
| - | Silk fibers from [https://en.wikipedia.org/wiki/Bombyx_mori Bombyx mori] (silkworm) have been utilized by mankind since ancient times due to their remarkable mechanical properties and comfort when woven into fabrics, with the earliest records dating to around 2700 BC <ref>DOI 10.1201/9781420015270</ref>. They are thermally comfortable, elastic, strong, and soft—properties that make silk highly sought after as a luxury fabric for garments. Silk is also biocompatible, making it applicable as a medical biomaterial for sutures, drug delivery systems, and scaffolds, where it plays a vital role in tissue regeneration<ref>DOI 10.1038/nprot.2011.379</ref>. | + | Silk fibers from [https://en.wikipedia.org/wiki/Bombyx_mori ''Bombyx mori''] (silkworm) have been utilized by mankind since ancient times due to their remarkable mechanical properties and comfort when woven into fabrics, with the earliest records dating to around 2700 BC <ref>DOI 10.1201/9781420015270</ref>. They are thermally comfortable, elastic, strong, and soft—properties that make silk highly sought after as a luxury fabric for garments. Silk is also biocompatible, making it applicable as a medical biomaterial for sutures, drug delivery systems, and scaffolds, where it plays a vital role in tissue regeneration<ref>DOI 10.1038/nprot.2011.379</ref>. |
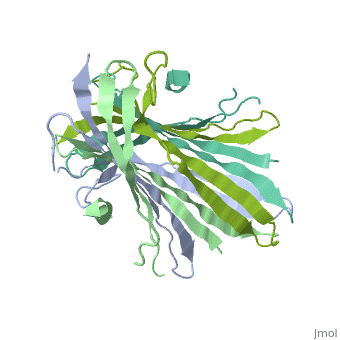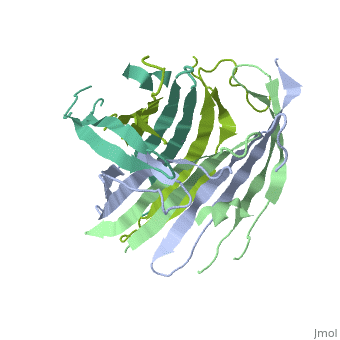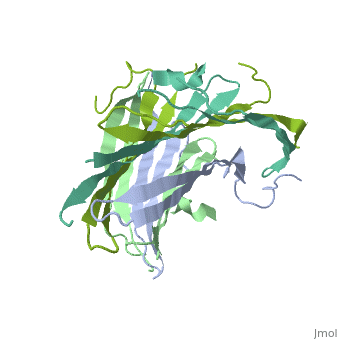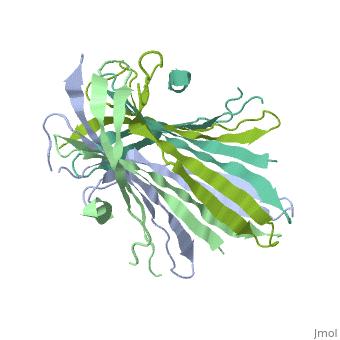
[[Fibroins|Fibroins]] are fibrous structural proteins composed of multiple subunits that assemble into high-strength materials like [https://en.wikipedia.org/wiki/Silk silk] and [https://en.wikipedia.org/wiki/Byssus byssal threads]. The exact composition varies by organism but typically features core fibroin filaments bundled within a sericin coating. These core filaments consist of three key components: a heavy chain (FibH), a light chain (FibL), and glycoproteins, all stabilized by disulfide bonds. The N-terminal domain of FibH (FibNT) plays a crucial role in the pH-dependent assembly of fibroin molecules during fiber formation <ref name="PDB">DOI 10.1016/j.jmb.2012.02.040</ref> (see [[#pH-Dependent Structural Transition|pH-Dependent Structural Transition]] section). | [[Fibroins|Fibroins]] are fibrous structural proteins composed of multiple subunits that assemble into high-strength materials like [https://en.wikipedia.org/wiki/Silk silk] and [https://en.wikipedia.org/wiki/Byssus byssal threads]. The exact composition varies by organism but typically features core fibroin filaments bundled within a sericin coating. These core filaments consist of three key components: a heavy chain (FibH), a light chain (FibL), and glycoproteins, all stabilized by disulfide bonds. The N-terminal domain of FibH (FibNT) plays a crucial role in the pH-dependent assembly of fibroin molecules during fiber formation <ref name="PDB">DOI 10.1016/j.jmb.2012.02.040</ref> (see [[#pH-Dependent Structural Transition|pH-Dependent Structural Transition]] section). | ||
| Line 12: | Line 12: | ||
== Biological production == | == Biological production == | ||
| - | Bombyx mori fifth-instar larvae produce silk proteins in specialized silk glands, where synthesis is spatially organized - fibroins (FibH, FibL, and glycoproteins) are produced in the posterior silk gland while sericins are synthesized in the middle section. Both components are stored in the lumen of the middle silk gland prior to fiber formation. The spinning process is mediated by precise pH and ion concentration gradients along the anterior silk gland. As the silk proteins encounter this gradient, the FibNT domain undergoes a critical conformational transition from random coil to β-sheet structure. This pH-dependent structural change, driven by protonation of key acidic residues, confers stability and strength to the fiber while enabling the final assembly of mature silk<ref name="PDB">DOI 10.1016/j.jmb.2012.02.040</ref>. | + | ''Bombyx mori'' fifth-instar larvae produce silk proteins in specialized silk glands, where synthesis is spatially organized - fibroins (FibH, FibL, and glycoproteins) are produced in the posterior silk gland while sericins are synthesized in the middle section. Both components are stored in the lumen of the middle silk gland prior to fiber formation. The spinning process is mediated by precise pH and ion concentration gradients along the anterior silk gland. As the silk proteins encounter this gradient, the FibNT domain undergoes a critical conformational transition from random coil to β-sheet structure. This pH-dependent structural change, driven by protonation of key acidic residues, confers stability and strength to the fiber while enabling the final assembly of mature silk<ref name="PDB">DOI 10.1016/j.jmb.2012.02.040</ref>. |
== Basic structure == | == Basic structure == | ||
Revision as of 16:50, 19 June 2025
FibNT (3UA0)
| |||||||||||
References
- ↑ doi: https://dx.doi.org/10.1201/9781420015270
- ↑ doi: https://dx.doi.org/10.1038/nprot.2011.379
- ↑ 3.0 3.1 He YX, Zhang NN, Li WF, Jia N, Chen BY, Zhou K, Zhang J, Chen Y, Zhou CZ. N-Terminal Domain of Bombyx mori Fibroin Mediates the Assembly of Silk in Response to pH Decrease. J Mol Biol. 2012 Mar 1. PMID:22387468 doi:10.1016/j.jmb.2012.02.040