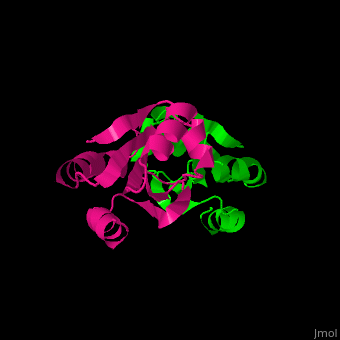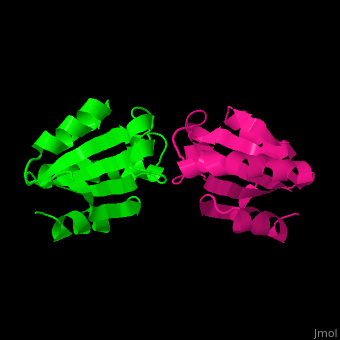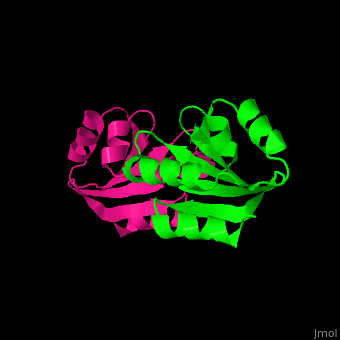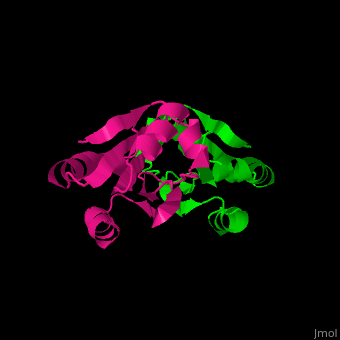We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Thioredoxin
From Proteopedia
(Difference between revisions)
| Line 40: | Line 40: | ||
== Structural highlights == | == Structural highlights == | ||
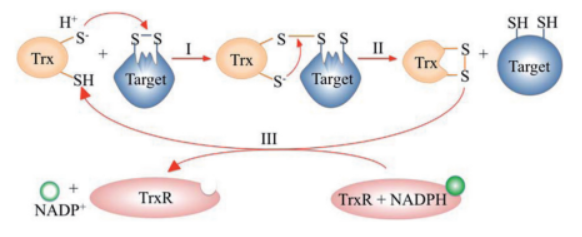
| + | The <scene name='43/430885/Cv/4'>active site motif Cys-Gly-Pro-Cys</scene> is involved in the reduction of disulfide bonds in proteins<ref>Åslund F et al. (1997). J Biol Chem. 272(48):30780–30786.</ref>. | ||
| + | Unlike many other thioredoxins, the human cytoplasmic thioredoxin has three cysteine residues (Cys 62, Cys 69, Cys 73) additional to the active site <scene name='43/430885/Cv/2'>Cys 32 and Cys 35</scene>. | ||
| + | The human cytoplasmic thioredoxin crystal structure reveals a homodimer with <scene name='43/430885/Cv/2'>Cys 73 forming an intermolecular disulfide bridge</scene>. | ||
| - | + | <StructureSection load='1ert' size='350' side='right' caption='Human thioredoxin (PDB entry 1ERT)' scene='43/430885/Cv/2'/> | |
| - | + | ||
| - | <StructureSection load='1ert' size='350' side='right' caption='Human thioredoxin (PDB entry 1ERT)' scene='43/430885/Cv/ | + | |
== 3D Structures of Thioredoxin == | == 3D Structures of Thioredoxin == | ||
Revision as of 19:28, 30 June 2025
| |||||||||||
References
- ↑ Oliveira LO. (2010). Caracterização de tiorredoxinas de fungos filamentosos como alvos moleculares para drogas antifúngicas. Tese de doutorado, Universidade de São Paulo.
- ↑ Laurent TC, Moore EC, Reichard P. (1964). J Biol Chem. 239(10):3436–3444.
- ↑ Netto LES et al. (2015). Free Radic Biol Med. 89:60–75.
- ↑ Holmgren A. (2000). Antioxid Redox Signal. 2(4):811–820.
- ↑ Sies H. (1985). Oxidative stress: introductory remarks. Academic Press.
- ↑ Yamada G et al. (2003). J Hepatol. 38(1):32–38.
- ↑ Arnér ESJ, Holmgren A. (2006). Semin Cancer Biol. 16(6):420–426.
- ↑ Åslund F et al. (1997). J Biol Chem. 272(48):30780–30786.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Laura Maria Batista Leal, Joel L. Sussman