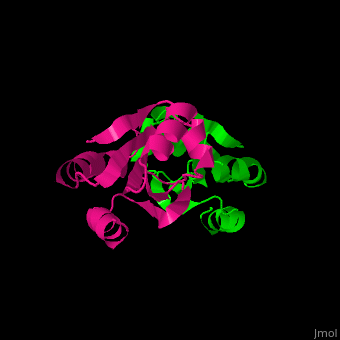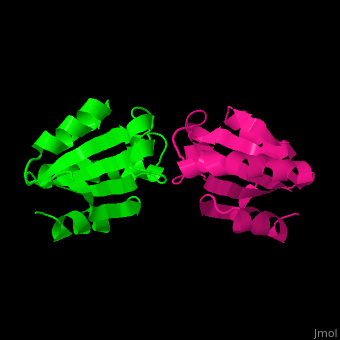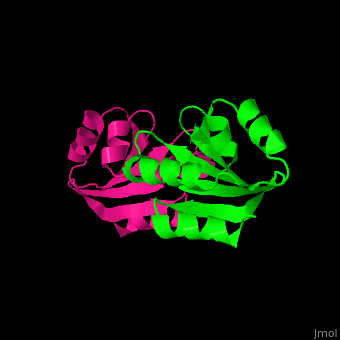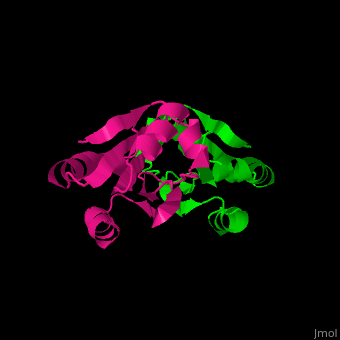Function
In response to the toxicity of reactive oxygen species (ROS), pathogenic organisms such as fungi possess enzymatic defense mechanisms. One such enzyme is Thioredoxin Reductase (TrxR), which plays essential roles in several biological processes including DNA synthesis, redox signaling, and oxidative stress response. Inhibiting TrxR can weaken pathogens, which may have significant implications for public health and agriculture.
Notably, fungal TrxRs are structurally and biochemically distinct from those found in mammals — a desirable feature for drug development efforts[1]. Furthermore, fungal TrxRs do not reduce the host thioredoxin, supporting their relevance as selective drug targets.
The Thioredoxin System
The thioredoxin system is composed of two main proteins: Thioredoxin (Trx) and Thioredoxin Reductase (TrxR), along with NADPH as a cofactor. This system functions primarily by reducing antioxidant proteins such as Peroxiredoxins (Prx) and Methionine Sulfoxide Reductases. It is also involved in other redox regulatory functions.
Historically, Trxs were the first enzymes identified as responsible for reducing ribonucleotides to deoxyribonucleotides in *Escherichia coli*, an essential step in DNA synthesis[2].
Mechanism of Action
TrxR catalyzes the reduction of the disulfide bond in the active site of Trx, enabling Trx to catalytically reduce target proteins. TrxR is a flavoprotein, using NADPH as the terminal electron donor.
The mechanism proceeds in sequential thiol-disulfide exchange reactions:
- **Step I:** The N-terminal cysteine of Trx attacks a disulfide bond in the target protein, forming a mixed disulfide intermediate.
- **Step II:** The C-terminal cysteine of Trx resolves the intermediate, releasing the reduced protein and generating oxidized Trx.
- **Step III:** TrxR recycles Trx using electrons from NADPH[3].
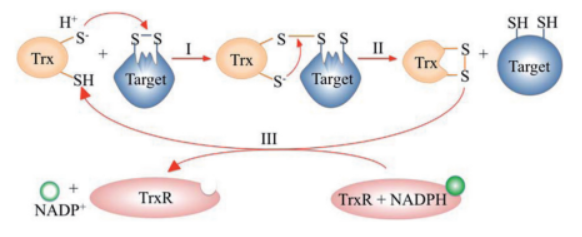
Figure 1: Catalytic cycle of the thioredoxin system. Adapted from Netto et al., 2015.
Thioredoxin (Trx) is an enzyme which facilitates, in its reduced form, the reduction of proteins by cysteine thiol-disulfide exchange[4]. They contain a CXXC motif.
- Trx-1 is a mammalian cellular protein.
- Trx-2 is mitochondria-specific.
- Trx C,M,X,Y are found in prokaryotes.
- Trx F,H,O are found in eukaryotes.
There are a range of strategies used by the host organism in an attempt to defend itself against pathogen invasion. Among these is the release of oxidants, such as reactive oxygen species (ROS), which at defined physiological concentrations act as signaling messengers. However, in supraphysiological concentrations, their reactivity has deleterious cellular consequences, causing damage to macromolecules such as proteins, lipids and DNA, thus impairing the system's homeostasis[5]. It is therefore an alternative used to inhibit the pathogen and prevent infection of the organism.
Relevance
Serum Trx level is a predictor of steatohepatitis[6].
Disease
Trx is involved in a wide range of human diseases and conditions including cancer, viral diseases, aging, cardiac conditions and more[7].
Structural highlights
The is involved in the reduction of disulfide bonds.
Unlike many other thioredoxins, the human thioredoxin has additional cysteines, including , which forms an intermolecular disulfide bridge in the homodimeric structure.
3D Structures of Thioredoxin
Thioredoxin 3D structures