Journal:Acta Cryst F:S2053230X25007034
From Proteopedia
(Difference between revisions)

| Line 10: | Line 10: | ||
The 1.99-Å crystal structure of HNL6V reveals the molecular basis for this engineered transformation. The seven substitutions induced <scene name='10/1087242/Ca_dist_HNL6V_to_HbHNL_all/1'>subtle but systematic shifts throughout the protein</scene>, moving the catalytic atoms 0.2-0.8 Å closer to their positions in the target esterase while increasing flexibility in the lid domain—demonstrating that the mutations both repositioned key atoms and fine-tuned protein dynamics. This work demonstrates that evolutionary trajectories between related enzymes can be partially retraced through rational design, offering new strategies for engineering enzymes with intermediate or altered catalytic properties. | The 1.99-Å crystal structure of HNL6V reveals the molecular basis for this engineered transformation. The seven substitutions induced <scene name='10/1087242/Ca_dist_HNL6V_to_HbHNL_all/1'>subtle but systematic shifts throughout the protein</scene>, moving the catalytic atoms 0.2-0.8 Å closer to their positions in the target esterase while increasing flexibility in the lid domain—demonstrating that the mutations both repositioned key atoms and fine-tuned protein dynamics. This work demonstrates that evolutionary trajectories between related enzymes can be partially retraced through rational design, offering new strategies for engineering enzymes with intermediate or altered catalytic properties. | ||
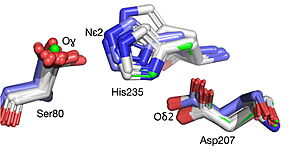
| - | [[Image:019 Fig 3a.triad label.jpg|thumb| | + | [[Image:019 Fig 3a.triad label.jpg|thumb|center|288px|Best fit overlay of the Cɑ positions of SABP2 structures (three structures, light blue carbons) and HbHNL structures (eighteen structures, white carbons) onto the structure of |
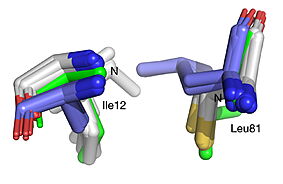
| - | HNL6V (green sticks). The catalytic atoms of the catalytic triad of this α/β-hydrolase fold, in HNL6V (Oɣ of S80, Nε2 of H235, Oδ2 of D207) overlay more closely with the corresponding atoms in the HbHNL structures (S80, H235, A207) than with the corresponding atoms in the SABP2 structures (S81, H238, D210). .]] [[Image:019_Fig_3b.oxyanion_label.jpg|thumb| | + | HNL6V (green sticks). The catalytic atoms of the catalytic triad of this α/β-hydrolase fold, in HNL6V (Oɣ of S80, Nε2 of H235, Oδ2 of D207) overlay more closely with the corresponding atoms in the HbHNL structures (S80, H235, A207) than with the corresponding atoms in the SABP2 structures (S81, H238, D210). .]] [[Image:019_Fig_3b.oxyanion_label.jpg|thumb|center|283px|Best fit overlay of the Cɑ positions of SABP2 structures (three structures, light blue carbons) and HbHNL structures (eighteen structures, white carbons) onto the structure of HNL6V (green sticks). The oxyanion hole amide nitrogen atoms of I12 and L81 in HNL6V overlay more closely with the corresponding atoms in HbHNL (I12, C81) than with the corresponding atoms in SABP2 (A13 and L82).]] |
<br> | <br> | ||
Revision as of 05:56, 13 August 2025
| |||||||||||
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.