Journal:Acta Cryst F:S2053230X25007034
From Proteopedia
(Difference between revisions)

| Line 7: | Line 7: | ||
The α/β-hydrolase fold superfamily serves as an excellent example of functional divergence—enzymes sharing nearly identical structures yet catalyzing different reactions. While most members of this family are esterases that hydrolyze ester bonds, hydroxynitrile lyases (HNLs) in this family have evolved to catalyze the reversible cleavage of cyanohydrins into aldehydes and hydrogen cyanide. Despite this functional divergence, both enzyme types retain the canonical Ser-His-Asp catalytic triad, raising questions about how active site geometry determines the catalytic mechanism. | The α/β-hydrolase fold superfamily serves as an excellent example of functional divergence—enzymes sharing nearly identical structures yet catalyzing different reactions. While most members of this family are esterases that hydrolyze ester bonds, hydroxynitrile lyases (HNLs) in this family have evolved to catalyze the reversible cleavage of cyanohydrins into aldehydes and hydrogen cyanide. Despite this functional divergence, both enzyme types retain the canonical Ser-His-Asp catalytic triad, raising questions about how active site geometry determines the catalytic mechanism. | ||
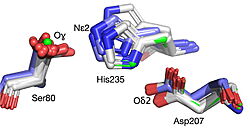
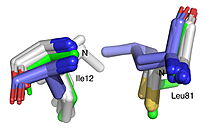
| - | The team focused on hydroxynitrile lyase from rubber trees (HbHNL) and attempted to make it more structurally similar to SABP2, an esterase from tobacco that shares 44% sequence identity. By introducing seven strategic mutations that match the corresponding positions in the target esterase, the researchers created <scene name='10/1087242/ | + | The team focused on hydroxynitrile lyase from rubber trees (HbHNL) and attempted to make it more structurally similar to SABP2, an esterase from tobacco that shares 44% sequence identity. By introducing seven strategic mutations that match the corresponding positions in the target esterase, the researchers created <scene name='10/1087242/019_Fig_2a_sticks_pse/1'>HNL6V, a hybrid enzyme</scene> that sits structurally between its parent and target enzymes. Six of the seven substitutions target the catalytic domain within or immediately adjacent to the active site, while the seventh targets the lid domain, also adjacent to the active site. |
The 1.99-Å crystal structure of HNL6V reveals the molecular basis for this engineered transformation. The seven substitutions induced <scene name='10/1087242/019_fig_02a_no_dist_png/3'>subtle but systematic shifts throughout the protein</scene>, moving the catalytic atoms 0.2-0.8 Å closer to their positions in the target esterase while increasing flexibility in the lid domain—demonstrating that the mutations both repositioned key atoms and fine-tuned protein dynamics. This work demonstrates that evolutionary trajectories between related enzymes can be partially retraced through rational design, offering new strategies for engineering enzymes with intermediate or altered catalytic properties. | The 1.99-Å crystal structure of HNL6V reveals the molecular basis for this engineered transformation. The seven substitutions induced <scene name='10/1087242/019_fig_02a_no_dist_png/3'>subtle but systematic shifts throughout the protein</scene>, moving the catalytic atoms 0.2-0.8 Å closer to their positions in the target esterase while increasing flexibility in the lid domain—demonstrating that the mutations both repositioned key atoms and fine-tuned protein dynamics. This work demonstrates that evolutionary trajectories between related enzymes can be partially retraced through rational design, offering new strategies for engineering enzymes with intermediate or altered catalytic properties. | ||
Current revision
| |||||||||||
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.