Kaushki Sharma- BI3323
From Proteopedia
(Difference between revisions)
| Line 29: | Line 29: | ||
<StructureSection load='9kkk' size='340' side='right'caption='Cryo-EM structure of human SLC22A6 (OAT1) in the apo-state, [[Resolution|resolution]] 3.85Å' scene=''> | <StructureSection load='9kkk' size='340' side='right'caption='Cryo-EM structure of human SLC22A6 (OAT1) in the apo-state, [[Resolution|resolution]] 3.85Å' scene=''> | ||
Classification: MEMBRANE PROTEIN | Classification: MEMBRANE PROTEIN | ||
| + | |||
| + | ===Introduction=== | ||
| + | |||
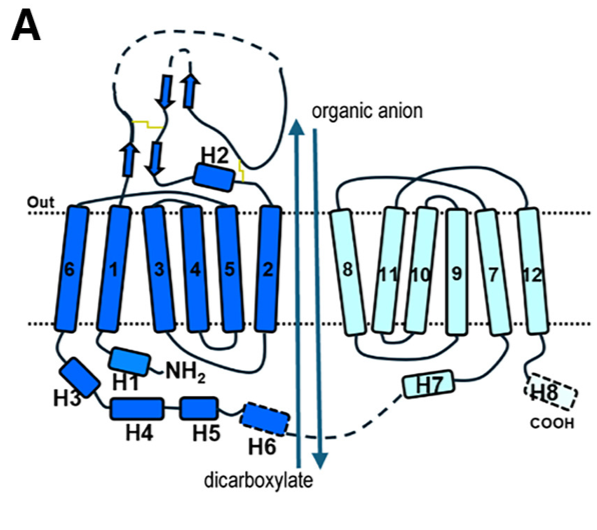
| + | Members of the organic anion transporter (OAT) family, including | ||
| + | OAT1, are expressed on the epithelial membrane of the kidney, | ||
| + | liver, brain, intestine, and placenta.<ref>Molecular cloning and characterization of a novel liver-specific transport protein https://doi.org/10.1242/jcs.107.4.1065</ref><ref>Molecular Cloning and Characterization of NKT, a Gene Product Related to the Organic Cation Transporter Family That Is Almost Exclusively Expressed in the Kidney https://doi.org/10.1074/jbc.272.10.6471</ref> OAT1 regulates the transport | ||
| + | of organic anion drugs from the blood into kidney epithelial | ||
| + | cells by utilizing the α-ketoglutarate (α-KG) gradient across the | ||
| + | membrane established by the tricarboxylic acid (TCA) cycle.<ref>Ingraham, L., Li, M., Renfro, J.L., Parker, S., Vapurcuyan, A., Hanna, I., and | ||
| + | Pelis, R.M. (2014). A plasma concentration of α-ketoglutarate influences | ||
| + | the kinetic interaction of ligands with organic anion transporter 1. Mol. | ||
| + | Pharmacol. 86, 86–95. https://doi.org/10.1124/mol.114.091777.</ref> <ref>Uwai, Y., Kawasaki, T., and Nabekura, T. (2017). D-Malate decreases renal | ||
| + | content of α-ketoglutarate, a driving force of organic anion transporters | ||
| + | OAT1 and OAT3, resulting in inhibited tubular secretion of phenolsulfonphthalein, | ||
| + | in rats. Biopharm. Drug Dispos. 38, 479–485. https://doi.org/10. | ||
| + | 1002/bdd.2089.</ref>OAT1 also plays a key role in excreting waste from organic drug metabolism and | ||
| + | contributes significantly to drug-drug interactions and drug disposition. However, the structural basis of specific | ||
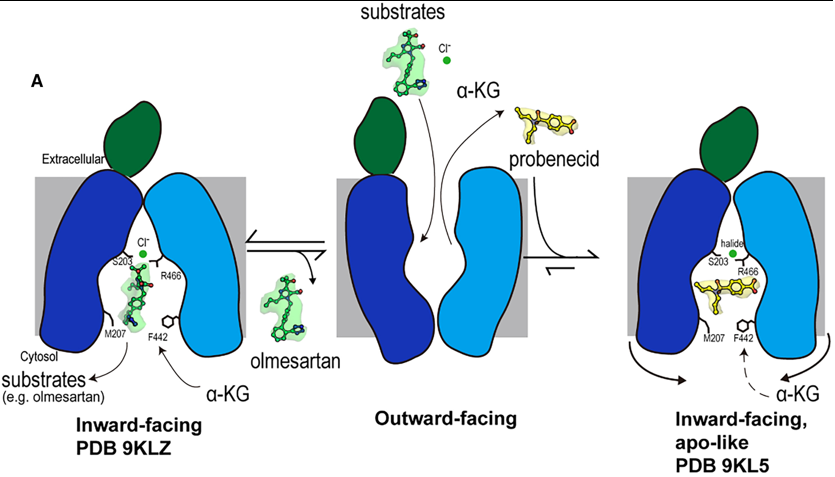
| + | substrate and inhibitor transport by human OAT1 (hOAT1) has remained elusive. Here are four | ||
| + | [[cryo-electron microscopy]] (cryo-EM) structures of hOAT1 in its inward-facing conformation: the apo | ||
| + | form, the substrate (olmesartan)-bound form with different anions, and the inhibitor (probenecid)-bound | ||
| + | form. | ||
Organism(s): Homo sapiens | Organism(s): Homo sapiens | ||
| Line 51: | Line 72: | ||
Reconstruction Method: SINGLE PARTICLE | Reconstruction Method: SINGLE PARTICLE | ||
| - | |||
| - | ===Introduction=== | ||
| - | |||
| - | Members of the organic anion transporter (OAT) family, including | ||
| - | OAT1, are expressed on the epithelial membrane of the kidney, | ||
| - | liver, brain, intestine, and placenta.<ref>Molecular cloning and characterization of a novel liver-specific transport protein https://doi.org/10.1242/jcs.107.4.1065</ref><ref>Molecular Cloning and Characterization of NKT, a Gene Product Related to the Organic Cation Transporter Family That Is Almost Exclusively Expressed in the Kidney https://doi.org/10.1074/jbc.272.10.6471</ref> OAT1 regulates the transport | ||
| - | of organic anion drugs from the blood into kidney epithelial | ||
| - | cells by utilizing the α-ketoglutarate (α-KG) gradient across the | ||
| - | membrane established by the tricarboxylic acid (TCA) cycle.<ref>Ingraham, L., Li, M., Renfro, J.L., Parker, S., Vapurcuyan, A., Hanna, I., and | ||
| - | Pelis, R.M. (2014). A plasma concentration of α-ketoglutarate influences | ||
| - | the kinetic interaction of ligands with organic anion transporter 1. Mol. | ||
| - | Pharmacol. 86, 86–95. https://doi.org/10.1124/mol.114.091777.</ref> <ref>Uwai, Y., Kawasaki, T., and Nabekura, T. (2017). D-Malate decreases renal | ||
| - | content of α-ketoglutarate, a driving force of organic anion transporters | ||
| - | OAT1 and OAT3, resulting in inhibited tubular secretion of phenolsulfonphthalein, | ||
| - | in rats. Biopharm. Drug Dispos. 38, 479–485. https://doi.org/10. | ||
| - | 1002/bdd.2089.</ref>OAT1 also plays a key role in excreting waste from organic drug metabolism and | ||
| - | contributes significantly to drug-drug interactions and drug disposition. However, the structural basis of specific | ||
| - | substrate and inhibitor transport by human OAT1 (hOAT1) has remained elusive. Here are four | ||
| - | [[cryo-electron microscopy]] (cryo-EM) structures of hOAT1 in its inward-facing conformation: the apo | ||
| - | form, the substrate (olmesartan)-bound form with different anions, and the inhibitor (probenecid)-bound | ||
| - | form. | ||
===Cryo-EM structure of hOAT1=== | ===Cryo-EM structure of hOAT1=== | ||
Revision as of 18:02, 30 November 2025
Interactive 3D Complement in Proteopedia
|
| |
|
Cryo-EM structures of human OAT1 reveal drug binding and inhibition mechanisms[1]. | |
|
Cell Volume 33, Issue 11, P1856-1866.E5, November 06, 2025 |
Structure Tour
| |||||||||||