We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
TRPC1 TRPC4 8WPL BI3323 Aug2025
From Proteopedia
(Difference between revisions)
(added the rest of the images) |
(final changes) |
||
| Line 17: | Line 17: | ||
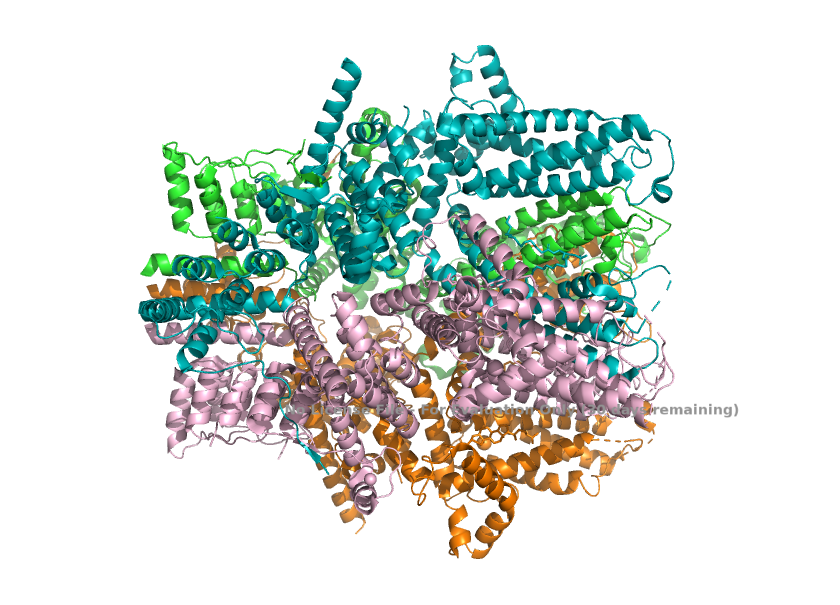
| - | == Structural highlights ==1. The channel, when TRPC1 is incorporated, loses the 4-fold symmetry with one TRPC1 and three TRPC4. This change in arrangement breaks the symmetry of the pore and creates a different, asymmetric ion-conduction pathway. <ref>Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. ''Nature Structural & Molecular Biology'', 32(2):326–338. DOI: 10.1038/s41594-024-01408-1</ref>[[Image:Asymmetry_(TRPC1_TRPC4_Stoichiometry)_.png]] | + | == Structural highlights ==1. The channel, when TRPC1 is incorporated, loses the 4-fold symmetry with one TRPC1 and three TRPC4. This change in arrangement breaks the symmetry of the pore and creates a different, asymmetric ion-conduction pathway. <ref>Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. ''Nature Structural & Molecular Biology'', 32(2):326–338. DOI: 10.1038/s41594-024-01408-1</ref> |
| + | |||
| + | [[Image:Asymmetry_(TRPC1_TRPC4_Stoichiometry)_.png]] | ||
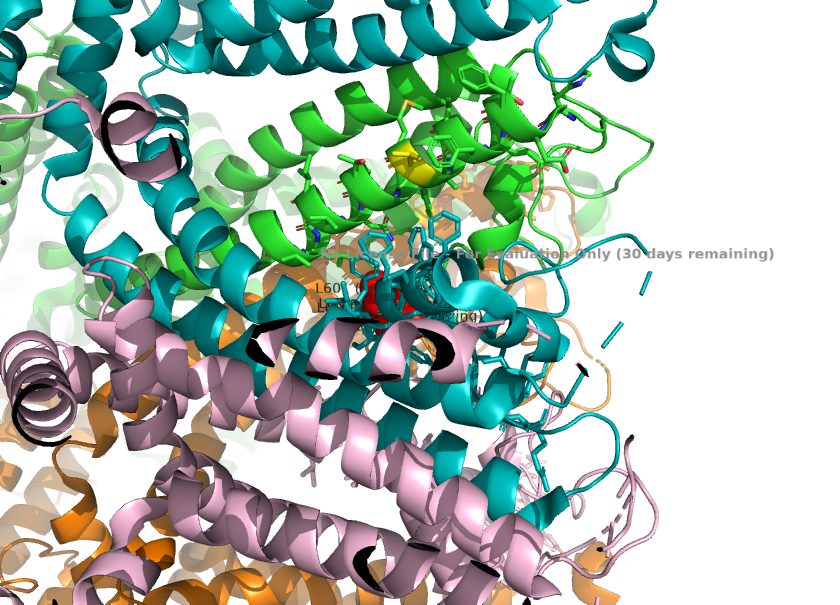
2. The selectivity filter loop(two maino acids longer than the corresponding loop in TRPC4) causes the loop to protrude further into the pore. L601 from TRPC1 is the key residue responsible for this, as it physically projects into the ion pathway, narrowing the pore radius. These change the channels' preference for monovalent channels. | 2. The selectivity filter loop(two maino acids longer than the corresponding loop in TRPC4) causes the loop to protrude further into the pore. L601 from TRPC1 is the key residue responsible for this, as it physically projects into the ion pathway, narrowing the pore radius. These change the channels' preference for monovalent channels. | ||
| - | <ref>Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. ''Nature Structural & Molecular Biology'', 32(2):326–338. DOI: 10.1038/s41594-024-01408-1</ref>[[Image:Selectivity_Filter_Narrowing_(L601)_.png]] | + | <ref>Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. ''Nature Structural & Molecular Biology'', 32(2):326–338. DOI: 10.1038/s41594-024-01408-1</ref> |
| - | 3. Calcium permeability is determined by the S6 helix present depeer in the pore. The TRPC1 subunit provides K639 here, which carries a positive charge in the central cavity of the pore. This creates an electropositive environment repelling calcium, which is also positively charged. <ref>Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. ''Nature Structural & Molecular Biology'', 32(2):326–338. DOI: 10.1038/s41594-024-01408-1</ref>[[Image:Calcium_Permeability_(K639_in_S6)_.png]] | + | |
| + | [[Image:Selectivity_Filter_Narrowing_(L601)_.png]] | ||
| + | |||
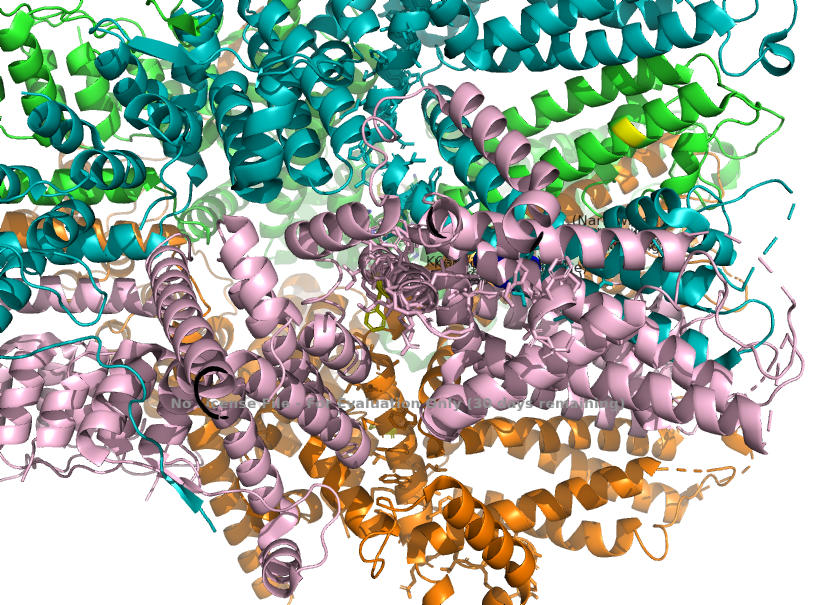
| + | 3. Calcium permeability is determined by the S6 helix present depeer in the pore. The TRPC1 subunit provides K639 here, which carries a positive charge in the central cavity of the pore. This creates an electropositive environment repelling calcium, which is also positively charged. <ref>Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. ''Nature Structural & Molecular Biology'', 32(2):326–338. DOI: 10.1038/s41594-024-01408-1</ref> | ||
| + | [[Image:Calcium_Permeability_(K639_in_S6)_.png]] | ||
Current revision
Overview of the TRPC1/TRPC4 Channel
| |||||||||||
References
- ↑ Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. Nature Structural & Molecular Biology, 32(2):326–338. DOI: 10.1038/s41594-024-01408-1
- ↑ Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. Nature Structural & Molecular Biology, 32(2):326–338. DOI: 10.1038/s41594-024-01408-1
- ↑ Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. Nature Structural & Molecular Biology, 32(2):326–338. DOI: 10.1038/s41594-024-01408-1
- ↑ Pani, B., Cornatzer, E. et al. (2006). Up-Regulation of Transient Receptor Potential Canonical 1 (TRPC1) following Sarco(endo)plasmic Reticulum Ca²⁺ ATPase 2 Gene Silencing Promotes Cell Survival: A Potential Role for TRPC1 in Darier's Disease. Molecular Biology of the Cell, 17(10):4446–4458.
- ↑ Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. Nature Structural & Molecular Biology, 32(2):326–338. DOI: 10.1038/s41594-024-01408-1
- ↑ Jeon, J., Moore, T. I., Sob, I. et al. (2025). TRPC4 regulates limbic behavior and neuronal development by stabilizing dendrite branches through actomyosin-driven integrin activation. PNAS, 122(33):e2511037ca122.
- ↑ Pani, B., Cornatzer, E. et al. (2006). Up-Regulation of Transient Receptor Potential Canonical 1 (TRPC1) following Sarco(endo)plasmic Reticulum Ca²⁺ ATPase 2 Gene Silencing Promotes Cell Survival: A Potential Role for TRPC1 in Darier's Disease. Molecular Biology of the Cell, 17(10):4446–4458.
- ↑ Jeon, J., Moore, T. I., Sob, I. et al. (2025). TRPC4 regulates limbic behavior and neuronal development by stabilizing dendrite branches through actomyosin-driven integrin activation. PNAS, 122(33):e2511037122.
- ↑ Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. Nature Structural & Molecular Biology, 32(2):326–338. DOI: 10.1038/s41594-024-01408-1
- ↑ Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. Nature Structural & Molecular Biology, 32(2):326–338. DOI: 10.1038/s41594-024-01408-1
- ↑ Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. Nature Structural & Molecular Biology, 32(2):326–338. DOI: 10.1038/s41594-024-01408-1
- ↑ Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. Nature Structural & Molecular Biology, 32(2):326–338. DOI: 10.1038/s41594-024-01408-1
- ↑ Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. Nature Structural & Molecular Biology, 32(2):326–338. DOI: 10.1038/s41594-024-01408-1
- ↑ Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. Nature Structural & Molecular Biology, 32(2):326–338. DOI: 10.1038/s41594-024-01408-1