Sandbox Aryan 20221057 BI3323-Aug2025
From Proteopedia
| Line 1: | Line 1: | ||
| - | + | <Structure load='8YNY' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | |
== Structure Overview == | == Structure Overview == | ||
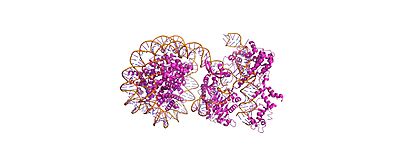
| - | Cas9-sgRNA ribonucleoprotein targets nucleosome **linker DNA** (PAM1/PAM28) and | + | Cas9-sgRNA ribonucleoprotein targets nucleosome **linker DNA** (PAM1/PAM28) and '''entry-exit regions''' (SHL6), avoiding tightly wrapped **core DNA** (SHL0-5). Native-PAGE on Widom 601 nucleosomes confirmed preferential cleavage at DNA ends where transient unwrapping occurs.[attached_file:1] |
The post-cleavage complex shows HNH/REC2 domains disordered, bridge helix absent, and target/non-target DNA strands cleaved—consistent with binary biochemical data.[web:2] | The post-cleavage complex shows HNH/REC2 domains disordered, bridge helix absent, and target/non-target DNA strands cleaved—consistent with binary biochemical data.[web:2] | ||
== PI Domain Interactions == | == PI Domain Interactions == | ||
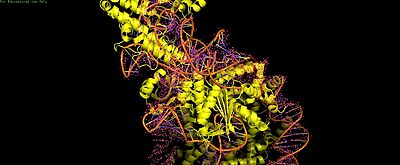
| - | Cas9's | + | Cas9's '''PI domain''' (residues ~1100-1368) makes multiple contacts: |
| - | - | + | - '''Histone tails''': Weak electrostatic interaction (non-essential for binding) |
| - | - | + | - '''PI edge (K1155)''': Lysine stabilizes post-cleavage complex via DNA phosphate backbone |
| - | - | + | - '''Core DNA loops (H1264/R1298/K1300)''': Nonspecific binding inhibits cleavage |
**Mutagenesis validation**: H1264A/R1298Q/K1300A mutants increase nucleosome binding AND cleavage efficiency both in vitro and rice callus genome editing.[attached_file:1] | **Mutagenesis validation**: H1264A/R1298Q/K1300A mutants increase nucleosome binding AND cleavage efficiency both in vitro and rice callus genome editing.[attached_file:1] | ||
== Dual Inhibition Mechanism == | == Dual Inhibition Mechanism == | ||
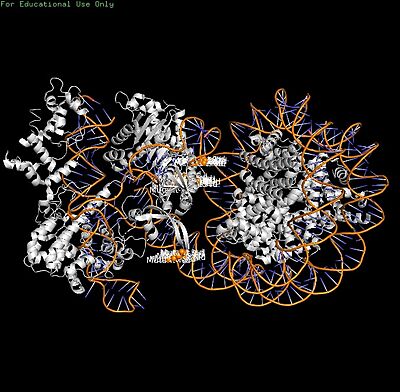
| - | 1. | + | 1. '''Access barrier''': Nucleosome DNA ends inflexible (SHL0-5), blocking Cas9 binding |
| - | 2. | + | 2. '''Motion restriction''': PI-core DNA trapping limits HNH/RuvC domain movements for cleavage |
| - | + | '''Entry/exit asymmetry''' from Widom601 sequence flexibility explains variable editing across chromatin contexts.[web:14] | |
== Implications == | == Implications == | ||
Revision as of 18:50, 30 November 2025
|
Contents |
Structure Overview
Cas9-sgRNA ribonucleoprotein targets nucleosome **linker DNA** (PAM1/PAM28) and entry-exit regions (SHL6), avoiding tightly wrapped **core DNA** (SHL0-5). Native-PAGE on Widom 601 nucleosomes confirmed preferential cleavage at DNA ends where transient unwrapping occurs.[attached_file:1]
The post-cleavage complex shows HNH/REC2 domains disordered, bridge helix absent, and target/non-target DNA strands cleaved—consistent with binary biochemical data.[web:2]
PI Domain Interactions
Cas9's PI domain (residues ~1100-1368) makes multiple contacts: - Histone tails: Weak electrostatic interaction (non-essential for binding) - PI edge (K1155): Lysine stabilizes post-cleavage complex via DNA phosphate backbone - Core DNA loops (H1264/R1298/K1300): Nonspecific binding inhibits cleavage
- Mutagenesis validation**: H1264A/R1298Q/K1300A mutants increase nucleosome binding AND cleavage efficiency both in vitro and rice callus genome editing.[attached_file:1]
Dual Inhibition Mechanism
1. Access barrier: Nucleosome DNA ends inflexible (SHL0-5), blocking Cas9 binding 2. Motion restriction: PI-core DNA trapping limits HNH/RuvC domain movements for cleavage
Entry/exit asymmetry from Widom601 sequence flexibility explains variable editing across chromatin contexts.[web:14]
Implications
Reveals Cas9's eukaryotic adaptation strategy and identifies **chromatin-optimized variants** for improved genome editing tools.[web:121]
BI3323-Aug2025