Photosystem II
From Proteopedia
(→Oxygen Evolution) |
(→Photosynthesis) |
||
| Line 5: | Line 5: | ||
Photosystem II, crystallized from the bacteria, ''Thermosynechococcus elongatus'', at 3.50 Å, is representative of the photosynthetic protein found in cyanobacteria and plants. The protein is associated with a variety of ligands. It is mainly composed of <scene name='Photosystem_II/Protein_only/1'>alpha helices</scene> and is a symmetrical <scene name='Photosystem_II/Psii_dimer/1'>dimer</scene>. Fifteen subunits are in each monomer, with multiple subunits associated with the oxygen evolving complex missing from this crystallization. Photosystem II is a membrane bound protein found in the thylakoid membrane of chloroplasts. <scene name='Photosystem_II/Hydrophobic_polar/1'>Polar and hydrophobic</scene> residues show orientation of the protein within the membrane. | Photosystem II, crystallized from the bacteria, ''Thermosynechococcus elongatus'', at 3.50 Å, is representative of the photosynthetic protein found in cyanobacteria and plants. The protein is associated with a variety of ligands. It is mainly composed of <scene name='Photosystem_II/Protein_only/1'>alpha helices</scene> and is a symmetrical <scene name='Photosystem_II/Psii_dimer/1'>dimer</scene>. Fifteen subunits are in each monomer, with multiple subunits associated with the oxygen evolving complex missing from this crystallization. Photosystem II is a membrane bound protein found in the thylakoid membrane of chloroplasts. <scene name='Photosystem_II/Hydrophobic_polar/1'>Polar and hydrophobic</scene> residues show orientation of the protein within the membrane. | ||
| - | == | + | ==Electron Transfer== |
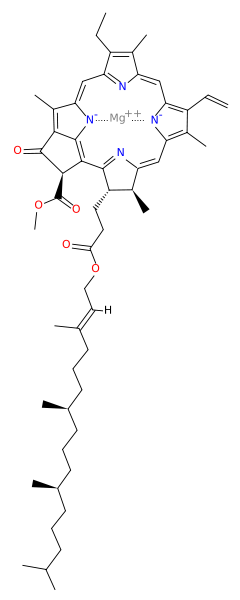
<scene name='Photosystem_II/Chlorophyll_green/1'>Chlorophyll</scene> surround Photosystem II and capture energy from sunlight, exciting electrons. Highly conjugated, these chlorophyll absorb visible light, along with as accessory light harvesting pigments such as <scene name='Photosystem_II/Betacarotene/1'>beta carotene</scene> that can absorb at other wavelengths. [[Image:b-car.svg.png|b-car.svg.png|right]] | <scene name='Photosystem_II/Chlorophyll_green/1'>Chlorophyll</scene> surround Photosystem II and capture energy from sunlight, exciting electrons. Highly conjugated, these chlorophyll absorb visible light, along with as accessory light harvesting pigments such as <scene name='Photosystem_II/Betacarotene/1'>beta carotene</scene> that can absorb at other wavelengths. [[Image:b-car.svg.png|b-car.svg.png|right]] | ||
Beta carotene also protects photosystem II by destroying reactive oxygen species that result from this photoexcitation. | Beta carotene also protects photosystem II by destroying reactive oxygen species that result from this photoexcitation. | ||
Electrons are passed from chlorophyll to <scene name='Photosystem_II/Pheophytin_purple/3'>pheophytin</scene>. Pheophytin are very similar to chlorophyll except they contain 2 H<sup>+</sup> instead of a Mg<sup>2+</sup> ion. From the pheophytin, electrons go to | Electrons are passed from chlorophyll to <scene name='Photosystem_II/Pheophytin_purple/3'>pheophytin</scene>. Pheophytin are very similar to chlorophyll except they contain 2 H<sup>+</sup> instead of a Mg<sup>2+</sup> ion. From the pheophytin, electrons go to | ||
| - | <scene name='Photosystem_II/Quinone_pink/3'>plastoquinones</scene>, which are reduced. Between each pair of quinones, an iron, in red, helps to transfer the electron between them. | + | <scene name='Photosystem_II/Quinone_pink/3'>plastoquinones</scene>, which are reduced. Between each pair of quinones, an iron, in red, helps to transfer the electron between them. [[Image:plastoquinone.jpg|right]] These plastoquinones eventually move to a plastoquinone pool which goes to another large protein subunit, photosystem I, the next step in photosynthesis. Eventually these electrons reduce NADP + to NADPH. |
==Oxygen Evolution== | ==Oxygen Evolution== | ||
Revision as of 21:15, 4 May 2008
|
Contents |
Background
Photosystem II, crystallized from the bacteria, Thermosynechococcus elongatus, at 3.50 Å, is representative of the photosynthetic protein found in cyanobacteria and plants. The protein is associated with a variety of ligands. It is mainly composed of and is a symmetrical . Fifteen subunits are in each monomer, with multiple subunits associated with the oxygen evolving complex missing from this crystallization. Photosystem II is a membrane bound protein found in the thylakoid membrane of chloroplasts. residues show orientation of the protein within the membrane.
Electron Transfer
surround Photosystem II and capture energy from sunlight, exciting electrons. Highly conjugated, these chlorophyll absorb visible light, along with as accessory light harvesting pigments such as that can absorb at other wavelengths.Beta carotene also protects photosystem II by destroying reactive oxygen species that result from this photoexcitation. Electrons are passed from chlorophyll to . Pheophytin are very similar to chlorophyll except they contain 2 H+ instead of a Mg2+ ion. From the pheophytin, electrons go to
, which are reduced. Between each pair of quinones, an iron, in red, helps to transfer the electron between them. These plastoquinones eventually move to a plastoquinone pool which goes to another large protein subunit, photosystem I, the next step in photosynthesis. Eventually these electrons reduce NADP + to NADPH.Oxygen Evolution
Another important facet of photosystem II is its ability to oxidize water to oxygen with its . These were shown to be cubane-like Mn3CaO4cluster linked to a fourth Mn by a mono-μ-oxo bridge. [1] Oxidation of water to leaves 2 H + on the lumenal side of the membrane, helping to establish the proton gradient essential for ATP synthesis in the CF1CF0-ATP sythase protein.
References
[1] Ferreira, K.N., Iverson, T.M., Maghlaoui, K., Barber, J., Iwata, S. "Architecture of the photosynthetic oxygen-evolving center." Science, March 19, 2004, 303 (5665), 1831-8.
[2] Garrett, R.H., Grisham, C.M. Biochemistry, 3rd Edition. Belmont, CA: Thomson Brooks/ Cole, 2005.
Proteopedia Page Contributors and Editors (what is this?)
Emily Forschler, Michal Harel, Ilan Samish, Alexander Berchansky, Eric Martz, Jaime Prilusky, Eran Hodis, Joel L. Sussman, David Canner, Karl Oberholser