This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
1ily
From Proteopedia
OCA (Talk | contribs)
(New page: 200px<br /><applet load="1ily" size="450" color="white" frame="true" align="right" spinBox="true" caption="1ily" /> '''Solution Structure of Ribosomal Protein L18 ...)
Next diff →
Revision as of 15:22, 20 November 2007
|
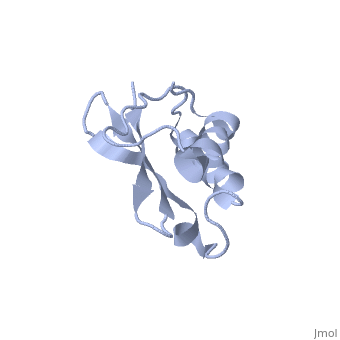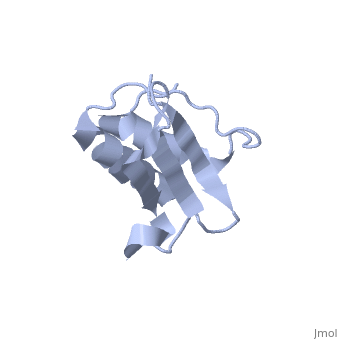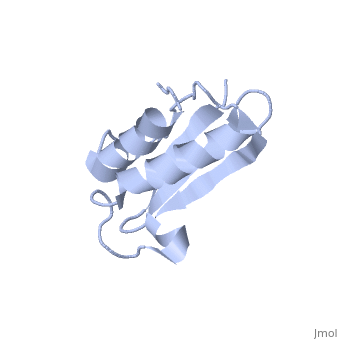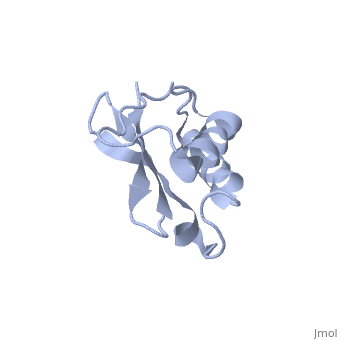
Solution Structure of Ribosomal Protein L18 of Thermus thermophilus
Overview
We have determined the solution structure of ribosomal protein L18 from, Thermus thermophilus. L18 is a 12.5 kDa protein of the large subunit of, the ribosome and binds to both 5 S and 23 S rRNA. In the uncomplexed state, L18 folds to a mixed alpha/beta globular structure with a long disordered, N-terminal region. We compared our high-resolution structure with, RNA-complexed L18 from Haloarcula marismortui and T. thermophilus to, examine RNA-induced as well as species-dependent structural differences., We also identified T. thermophilus S11 as a structural homologue and found, that the structures of the RNA-recognition sites are conserved. Important, features, for instance a bulge in the RNA-contacting beta-sheet, are, conserved in both proteins. We suggest that the L18 fold recognizes a, specific RNA motif and that the resulting RNA-protein-recognition module, is tolerant to variations in sequence.
About this Structure
1ILY is a Single protein structure of sequence from Thermus thermophilus. Full crystallographic information is available from OCA.
Reference
The solution structure of ribosomal protein L18 from Thermus thermophilus reveals a conserved RNA-binding fold., Woestenenk EA, Gongadze GM, Shcherbakov DV, Rak AV, Garber MB, Hard T, Berglund H, Biochem J. 2002 May 1;363(Pt 3):553-61. PMID:11964156
Page seeded by OCA on Tue Nov 20 17:29:35 2007