1ls8
From Proteopedia
OCA (Talk | contribs)
(New page: 200px<br /><applet load="1ls8" size="450" color="white" frame="true" align="right" spinBox="true" caption="1ls8" /> '''NMR structure of the unliganded Bombyx mori ...)
Next diff →
Revision as of 18:42, 20 November 2007
|
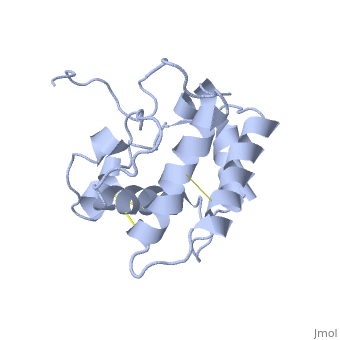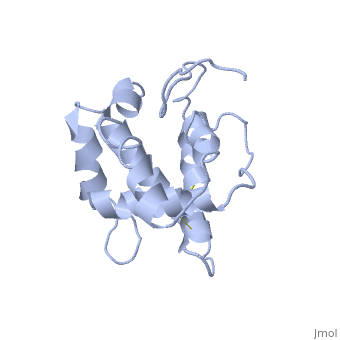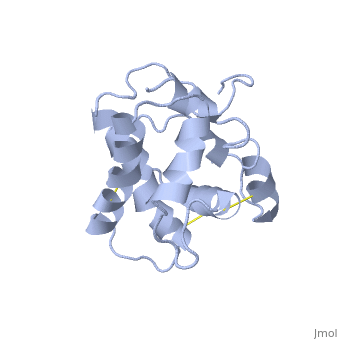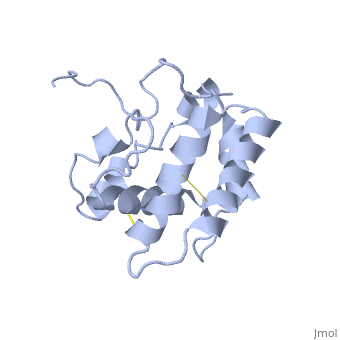
NMR structure of the unliganded Bombyx mori pheromone-binding protein at physiological pH
Overview
The nuclear magnetic resonance structure of the unliganded, pheromone-binding protein (PBP) from Bombyx mori at pH above 6.5, BmPBP(B), consists of seven helices with residues 3-8, 16-22, 29-32, 46-59, 70-79, 84-100, and 107-124, and contains the three disulfide, bridges 19-54, 50-108, and 97-117. This polypeptide fold encloses a large, hydrophobic cavity, with a sufficient volume to accommodate the natural, ligand bombykol. The polypeptide folds in free BmPBP(B) and in crystals of, a BmPBP-bombykol complex are nearly identical, indicating that the B-form, of BmPBP in solution represents the active conformation for ligand, binding.
About this Structure
1LS8 is a Single protein structure of sequence from Bombyx mori. Full crystallographic information is available from OCA.
Reference
NMR structure of the unliganded Bombyx mori pheromone-binding protein at physiological pH., Lee D, Damberger FF, Peng G, Horst R, Guntert P, Nikonova L, Leal WS, Wuthrich K, FEBS Lett. 2002 Nov 6;531(2):314-8. PMID:12417333
Page seeded by OCA on Tue Nov 20 20:49:51 2007