1qr0
From Proteopedia
OCA (Talk | contribs)
(New page: 200px<br /><applet load="1qr0" size="450" color="white" frame="true" align="right" spinBox="true" caption="1qr0, resolution 1.90Å" /> '''CRYSTAL STRUCTURE OF...)
Next diff →
Revision as of 22:47, 20 November 2007
|
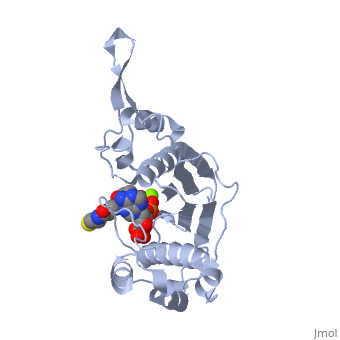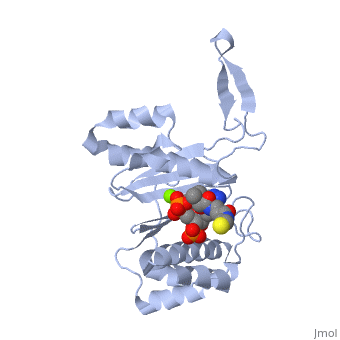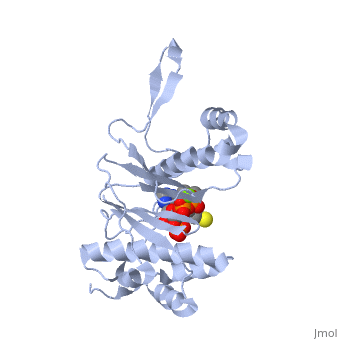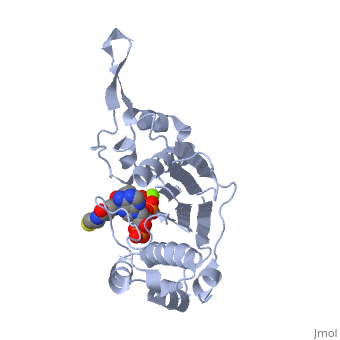
CRYSTAL STRUCTURE OF THE 4'-PHOSPHOPANTETHEINYL TRANSFERASE SFP-COENZYME A COMPLEX
Overview
The Bacillus subtilis Sfp protein activates the peptidyl carrier protein, (PCP) domains of surfactin synthetase by transferring the, 4'-phosphopantetheinyl moiety of coenzyme A (CoA) to a serine residue, conserved in all PCPs. Its wide PCP substrate spectrum renders Sfp a, biotechnologically valuable enzyme for use in combinatorial non-ribosomal, peptide synthesis. The structure of the Sfp-CoA complex determined at 1.8, A resolution reveals a novel alpha/beta-fold exhibiting an unexpected, intramolecular 2-fold pseudosymmetry. This suggests a similar fold and, dimerization mode for the homodimeric phosphopantetheinyl transferases, such as acyl carrier protein synthase. The active site of Sfp accommodates, a magnesium ion, which is complexed by the CoA pyrophosphate, the side, chains of three acidic amino acids and one water molecule. CoA is bound in, a fashion that differs in many aspects from all known CoA-protein complex, structures. The structure reveals regions likely to be involved in the, interaction with the PCP substrate.
About this Structure
1QR0 is a Single protein structure of sequence from Bacillus subtilis with MG and COA as ligands. Full crystallographic information is available from OCA.
Reference
Crystal structure of the surfactin synthetase-activating enzyme sfp: a prototype of the 4'-phosphopantetheinyl transferase superfamily., Reuter K, Mofid MR, Marahiel MA, Ficner R, EMBO J. 1999 Dec 1;18(23):6823-31. PMID:10581256
Page seeded by OCA on Wed Nov 21 00:54:28 2007