User:Tom Gluick/glutamine synthetase
From Proteopedia
(→Introduction) |
m (→Introduction) |
||
| Line 5: | Line 5: | ||
Glutamine synthetase appears to come in three major phylogenetically related classes of structures[http://pfam.sanger.ac.uk/family?acc=PF00120]. The GS-I class is found in many prokaryotes consists of twelve identical subunits arranged as two stacked and interlocking hexameric rings. The crystal structure of the Salmonella typhimurium enzyme has been solved and published in 1989, and its regulation is a subject of textbook discussion[http://www.ebi.ac.uk/pdbsum/2gls]. Briefly, the enzyme is regulated precisely via feedback inhibition by metabolic products such as glycine, alanine, carbamoyl phosphate, CTP and five others, and covalent modification via the adenylylation of tyrosyl residue. Crystal structures for Salmonella typhimurium glutamine synthetase illustrate adenylylated, unadenylylated, and ligand bound states (1lgr[http://www.proteopedia.org/wiki/index.php/1lgr], 2gls[http://www.proteopedia.org/wiki/index.php/2gls], 2lgo[http://www.proteopedia.org/wiki/index.php/2lgo], 1f1h[http://www.proteopedia.org/wiki/index.php/1f1h], 1fs2[http://www.proteopedia.org/wiki/index.php/1fs2] and 1fpy[http://www.proteopedia.org/wiki/index.php/1fpy]). Explaining how this enzyme works is the focus of your projects. <br/> | Glutamine synthetase appears to come in three major phylogenetically related classes of structures[http://pfam.sanger.ac.uk/family?acc=PF00120]. The GS-I class is found in many prokaryotes consists of twelve identical subunits arranged as two stacked and interlocking hexameric rings. The crystal structure of the Salmonella typhimurium enzyme has been solved and published in 1989, and its regulation is a subject of textbook discussion[http://www.ebi.ac.uk/pdbsum/2gls]. Briefly, the enzyme is regulated precisely via feedback inhibition by metabolic products such as glycine, alanine, carbamoyl phosphate, CTP and five others, and covalent modification via the adenylylation of tyrosyl residue. Crystal structures for Salmonella typhimurium glutamine synthetase illustrate adenylylated, unadenylylated, and ligand bound states (1lgr[http://www.proteopedia.org/wiki/index.php/1lgr], 2gls[http://www.proteopedia.org/wiki/index.php/2gls], 2lgo[http://www.proteopedia.org/wiki/index.php/2lgo], 1f1h[http://www.proteopedia.org/wiki/index.php/1f1h], 1fs2[http://www.proteopedia.org/wiki/index.php/1fs2] and 1fpy[http://www.proteopedia.org/wiki/index.php/1fpy]). Explaining how this enzyme works is the focus of your projects. <br/> | ||
| - | The human enzyme is classed as GS-II. The quaternary structure consists of ten identical subunits arranged as two pentameric rings stacked atop each other [http://www.ebi.ac.uk/pdbsum/2d3b. ] as shown in the Java Applet on the right hand side of the page. Glutamine synthetase is a key component in controlling ammonia concentrations, maintaining nitrogen balance among organs, acid-base homeostasis, gene regulation and signaling.[references] In ureoteles, glutamine serves as a nontoxic shuttle of excess ammonia as its transported from tissues not capable of processing ammonia further through the blood to the liver where amide nitrogen eventually ends up in urea. The ecology of neuron and astrocyte involves regulating the concentration of glutamate by...[]. Glutamine is involved in ...[] Disruptions in glutamine homeostasis ...brought about through .... cause ... organ failure and death.. various organs. Although extremely rare and only seen in the children of consnaguionoius couples, that defects in GLUL ( the gene encoding for gluatmine synthetase) causes a congenital disorder leading to brain malformation and death in neonates[http://ca.expasy.org/cgi-bin/niceprot.pl?P15104].<br/> | + | The human enzyme is classed as GS-II. The quaternary structure consists of ten identical subunits arranged as two pentameric rings stacked atop each other [http://www.ebi.ac.uk/pdbsum/2d3b. ] as shown in the Java Applet on the right hand side of the page. Glutamine synthetase is a key component in controlling ammonia concentrations, maintaining nitrogen balance among organs, acid-base homeostasis, gene regulation and signaling.[references] In ureoteles, glutamine serves as a nontoxic shuttle of excess ammonia as its transported from tissues not capable of processing ammonia further through the blood to the liver where amide nitrogen eventually ends up in urea. The ecology of neuron and astrocyte involves regulating the concentration of glutamate by...[]. |
| + | |||
| + | Glutamine is involved in ...[] | ||
| + | |||
| + | Glutamine homeostasis is maintained in part via regulating transcription and GS protein degradation. <ref> Labow, B. I., et. al., Mechanisms Governing the Expression of the Enzymes of Glutamine | ||
| + | Metabolism—Glutaminase and Glutamine Synthetase. J. Nutr. 2001 131: 2467S–2474S.</ref. | ||
| + | |||
| + | Disruptions in glutamine homeostasis ...brought about through .... cause ... organ failure and death.. various organs. Although extremely rare and only seen in the children of consnaguionoius couples, that defects in GLUL ( the gene encoding for gluatmine synthetase) causes a congenital disorder leading to brain malformation and death in neonates[http://ca.expasy.org/cgi-bin/niceprot.pl?P15104].<br/> | ||
In humans, glutamine concentrations are regulated via complex mechanisms and gluatmine is implicated in gene expression in a variety of organs. | In humans, glutamine concentrations are regulated via complex mechanisms and gluatmine is implicated in gene expression in a variety of organs. | ||
Revision as of 02:06, 12 October 2008
Introduction
| |||||||||
| 2qc8, resolution 2.60Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , , | ||||||||
| Gene: | GLUL, GLNS (Homo sapiens) | ||||||||
| Activity: | Glutamate--ammonia ligase, with EC number 6.3.1.2 | ||||||||
| Related: | 2ojw | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
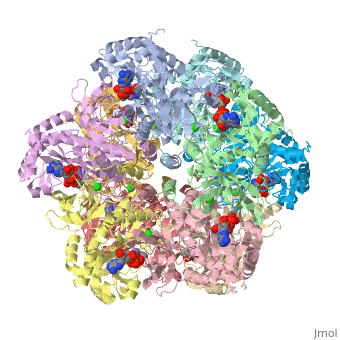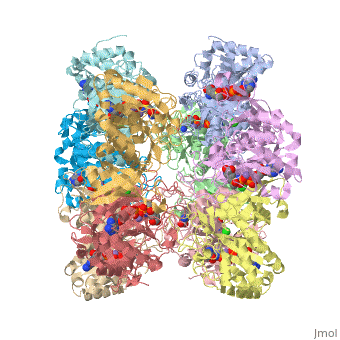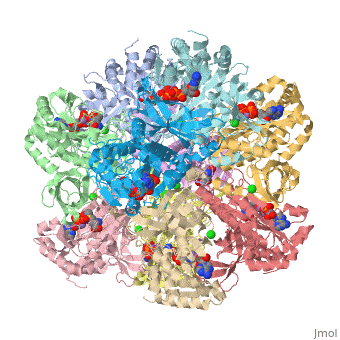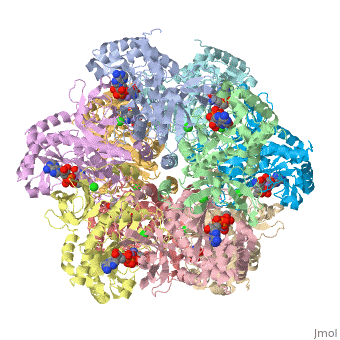
Glutamine synthetase is a key component in the regulation of the concentration of nitrogen containing compounds through out the phyla. The enzyme synthesizes glutamine from glutamate, ATP, and ammonium ion via a two step mechanism involving an glutamyl-P intermediate. The ATP provides the driving force for the reaction by esterifying glutamate's C-3 carboxyl group with the γ-phosphate that is later displaced by ammonia. Glutamine is incorporated into proteins, serves as an energy source, is involved in assimilating ammonia to be used in amino acid and nucleic acid synthesis.
Glutamine synthetase appears to come in three major phylogenetically related classes of structures[1]. The GS-I class is found in many prokaryotes consists of twelve identical subunits arranged as two stacked and interlocking hexameric rings. The crystal structure of the Salmonella typhimurium enzyme has been solved and published in 1989, and its regulation is a subject of textbook discussion[2]. Briefly, the enzyme is regulated precisely via feedback inhibition by metabolic products such as glycine, alanine, carbamoyl phosphate, CTP and five others, and covalent modification via the adenylylation of tyrosyl residue. Crystal structures for Salmonella typhimurium glutamine synthetase illustrate adenylylated, unadenylylated, and ligand bound states (1lgr[3], 2gls[4], 2lgo[5], 1f1h[6], 1fs2[7] and 1fpy[8]). Explaining how this enzyme works is the focus of your projects.
The human enzyme is classed as GS-II. The quaternary structure consists of ten identical subunits arranged as two pentameric rings stacked atop each other [9] as shown in the Java Applet on the right hand side of the page. Glutamine synthetase is a key component in controlling ammonia concentrations, maintaining nitrogen balance among organs, acid-base homeostasis, gene regulation and signaling.[references] In ureoteles, glutamine serves as a nontoxic shuttle of excess ammonia as its transported from tissues not capable of processing ammonia further through the blood to the liver where amide nitrogen eventually ends up in urea. The ecology of neuron and astrocyte involves regulating the concentration of glutamate by...[].
Glutamine is involved in ...[]
Glutamine homeostasis is maintained in part via regulating transcription and GS protein degradation. [1]