Green Fluorescent Protein
From Proteopedia
| Line 9: | Line 9: | ||
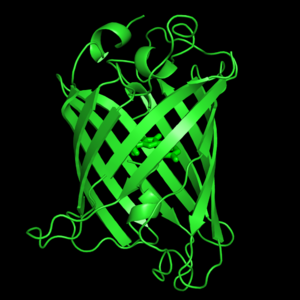
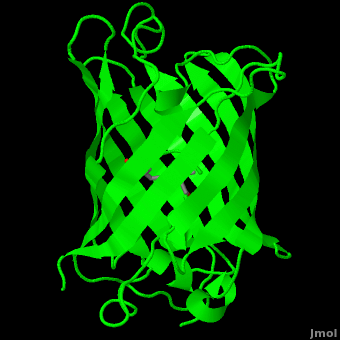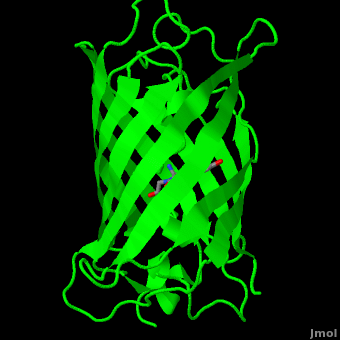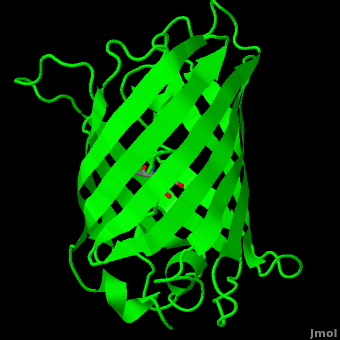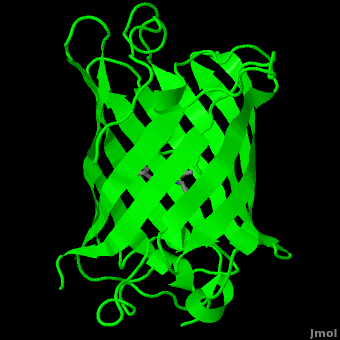
| - | The crystal structure of GFP <ref>Crystal structure of the Aequorea victoria green fluorescent protein., Ormö M, Cubitt AB, Kallio K, Gross LA, Tsien RY, Remington SJ, Science 1996 6;273(5280):1392-1395. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/8703075 8703075]</ref><ref>The molecular structure of green fluorescent protein., Yang F, Moss LG, Phillips GN Jr, Nat Biotechnol. 1996 Oct;14(10):1246-51. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/9631087 9631087]</ref> is an eleven-stranded anti-parallel beta- | + | The crystal structure of GFP <ref>Crystal structure of the Aequorea victoria green fluorescent protein., Ormö M, Cubitt AB, Kallio K, Gross LA, Tsien RY, Remington SJ, Science 1996 6;273(5280):1392-1395. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/8703075 8703075]</ref><ref>The molecular structure of green fluorescent protein., Yang F, Moss LG, Phillips GN Jr, Nat Biotechnol. 1996 Oct;14(10):1246-51. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/9631087 9631087]</ref> is <scene name='Green_Fluorescent_Protein/1ema_gfp_default/1'>an eleven-stranded anti-parallel beta- |
| - | barrel, threaded by an alpha-helix, running up along the axis of the cylinder. | + | barrel, threaded by an alpha-helix, running up along the axis of the cylinder</scene>. |
The chromophore is in the distorted alpha-helix that runs along the axis of the can, close to the center of the can-like cylinder. | The chromophore is in the distorted alpha-helix that runs along the axis of the can, close to the center of the can-like cylinder. | ||
Revision as of 04:49, 21 November 2008
Contents |
Background
Osamu Shimomura, Martin Chalfie and Roger Y. Tsien shared the 2008 Nobel Prize in Chemistry for their for the discovery and development of the green fluorescent protein, GFP.
GFP is small protein (21 kDa) and does not require cofactors to become fluorescent.
Structure
| |||||||
The crystal structure of GFP [1][2] is .
The chromophore is in the distorted alpha-helix that runs along the axis of the can, close to the center of the can-like cylinder.
The chromophore is formed from the tripeptide motif Ser65-Tyr66-Gly67 after translation and folding of GFP. As the GFP protein folds into its native conformation, these three amino acids are forced into a sharp turn, greatly favoring a nucleophilic attack of the amide of Gly67 on the carbonyl of Ser65, leading to imidazolinone formation by cyclization and dehydration. At this point, GFP is not fluorescent;however, in the presence of molecular oxygen, the α–β bond of residue 66 is subsequently dehydrogenated into conjugation with the imidazolinone, which results in maturation of the GFP chromophore to its fluorescent form [3][4].
You can take a close look at the of GFP in the PDB entry 1ema.
The chromophore
Reference for the Structure
Crystal structure of the Aequorea victoria green fluorescent protein., Ormo M, Cubitt AB, Kallio K, Gross LA, Tsien RY, Remington SJ, Science. 1996 Sep 6;273(5280):1392-5. PMID:8703075
Related Structures and Topics
- 1gfl Aequorea victoria Green Fluorescent Protein
- 1b9c Aequorea victoria Green Fluorescent Protein Mutant F99s, M153t And V163a
Notes and Literature References
- ↑ Crystal structure of the Aequorea victoria green fluorescent protein., Ormö M, Cubitt AB, Kallio K, Gross LA, Tsien RY, Remington SJ, Science 1996 6;273(5280):1392-1395. PMID:8703075
- ↑ The molecular structure of green fluorescent protein., Yang F, Moss LG, Phillips GN Jr, Nat Biotechnol. 1996 Oct;14(10):1246-51. PMID:9631087
- ↑ Wavelength mutations and posttranslational autoxidation of green fluorescent protein., Heim R, Prasher DC, Tsien RY, Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12501-4. PMID:7809066
- ↑ Understanding, improving and using green fluorescent proteins. Cubitt AB, Heim R, Adams SR, Boyd AE, Gross LA, Tsien RY, Trends Biochem Sci. 1995 Nov;20(11):448-55.PMID:8578587
Additional information on green fluorescent protein
- Roger Y. Tsien (1998) The Green Fluorescent Protein. Annual Review of Biochemistry 67, 509-544.
- [The GFP site] by Marc Zimmer, Ph. D., at Connecticut College, who authored [Glowing Genes: A Revolution In Biotechnology].
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Wayne Decatur, Karsten Theis, Eran Hodis, Laura Carbone, Karl Oberholser, Mark Hoelzer, Joel L. Sussman, Alexander Berchansky, Jaime Prilusky, Marius Mihasan, Joseph M. Steinberger, David Canner