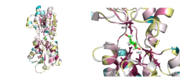User:Mariel Feliciano
From Proteopedia
| Line 1: | Line 1: | ||
| - | |||
== Glucose/galactose-binding protein == | == Glucose/galactose-binding protein == | ||
| Line 5: | Line 4: | ||
[[Image:superposition.png|thumb|Figure 1 Superposition of open and closed conformations of GGBP]] | [[Image:superposition.png|thumb|Figure 1 Superposition of open and closed conformations of GGBP]] | ||
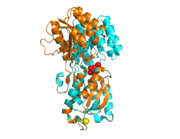
| + | [[Image:consurf_images.png|thumb|Figure 2 Structures color-coded from least(cyan) to most(maroon) conserved residues as defined by Consurf]] | ||
| - | Glucose/galactose-binding protein (GGBP) from | + | Glucose/galactose-binding protein (GGBP) from |
''[http://en.wikipedia.org/wiki/E._coli E. coli]'' is found in the | ''[http://en.wikipedia.org/wiki/E._coli E. coli]'' is found in the | ||
[http://en.wikipedia.org/wiki/Periplasm periplasm] of the | [http://en.wikipedia.org/wiki/Periplasm periplasm] of the | ||
| Line 16: | Line 16: | ||
<scene name='User:Mariel_Feliciano/Hinges_red/1'>"hinges"</scene> because on their role in the opening/closing mechanism of the protein. The protein exists in two conformations: glucose-bound (closed) and unbound (open) (Figure 1). Upon ligand binding, changes in the torsional angles of the amino acids that compose the hinges produce a conformational change between the two domains that stabilize the closed conformation. Analysis of the structure with | <scene name='User:Mariel_Feliciano/Hinges_red/1'>"hinges"</scene> because on their role in the opening/closing mechanism of the protein. The protein exists in two conformations: glucose-bound (closed) and unbound (open) (Figure 1). Upon ligand binding, changes in the torsional angles of the amino acids that compose the hinges produce a conformational change between the two domains that stabilize the closed conformation. Analysis of the structure with | ||
[http://consurf.tau.ac.il/ consurf] | [http://consurf.tau.ac.il/ consurf] | ||
| - | <ref>Landau, M., Mayrose, I., Rosenberg, Y., Glaser, F., Martz, E., Pupko, T., et al. (2005). ConSurf 2005: The projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Research, 33(Web Server issue), W299-302. </ref> shows that amino acids that line the | + | <ref>Landau, M., Mayrose, I., Rosenberg, Y., Glaser, F., Martz, E., Pupko, T., et al. (2005). ConSurf 2005: The projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Research, 33(Web Server issue), W299-302. </ref>(Figure 2) shows that amino acids that line the |
<scene name='User:Mariel_Feliciano/Binding_site_b/1'>binding site</scene> and amino acids that compose the hinges are the most conserved. Despite its homology with other sugar-binding proteins like ribose-binding protein (RBP), the changes in torsional angles of the amino acids that compose the hinges and trigger the conformational changes between domains differ in both proteins. One of the structures reported by Borrok, M.J., Kiessling, L.L., and Forest, K.T. | <scene name='User:Mariel_Feliciano/Binding_site_b/1'>binding site</scene> and amino acids that compose the hinges are the most conserved. Despite its homology with other sugar-binding proteins like ribose-binding protein (RBP), the changes in torsional angles of the amino acids that compose the hinges and trigger the conformational changes between domains differ in both proteins. One of the structures reported by Borrok, M.J., Kiessling, L.L., and Forest, K.T. | ||
<ref name="primary citation">Borrok, M. J., Kiessling, L. L., & Forest, K. T. (2007). Conformational changes of glucose/galactose-binding protein illuminated by open, unliganded, and ultra-high-resolution ligand-bound structures. Protein Science : A Publication of the Protein Society, 16(6), 1032-1041. </ref> | <ref name="primary citation">Borrok, M. J., Kiessling, L. L., & Forest, K. T. (2007). Conformational changes of glucose/galactose-binding protein illuminated by open, unliganded, and ultra-high-resolution ligand-bound structures. Protein Science : A Publication of the Protein Society, 16(6), 1032-1041. </ref> | ||
Revision as of 16:44, 28 November 2008
Glucose/galactose-binding protein
|
Glucose/galactose-binding protein (GGBP) from E. coli is found in the periplasm of the bacterium. This protein is involved in the transport of glucoseas well as the chemotaxis toward it. It is composed of two Rossman folds separated by three short segments of amino acids termed the because on their role in the opening/closing mechanism of the protein. The protein exists in two conformations: glucose-bound (closed) and unbound (open) (Figure 1). Upon ligand binding, changes in the torsional angles of the amino acids that compose the hinges produce a conformational change between the two domains that stabilize the closed conformation. Analysis of the structure with consurf [1](Figure 2) shows that amino acids that line the and amino acids that compose the hinges are the most conserved. Despite its homology with other sugar-binding proteins like ribose-binding protein (RBP), the changes in torsional angles of the amino acids that compose the hinges and trigger the conformational changes between domains differ in both proteins. One of the structures reported by Borrok, M.J., Kiessling, L.L., and Forest, K.T. [2] (PDB ID 2FW0) is the first crystal structure of the protein in its open conformation. The other structure reported by these authors [2] (PDB ID 2FVY) is an ultra-high resolution (0.95Å) crystal structure of the glucose-bound protein.
References
- ↑ Landau, M., Mayrose, I., Rosenberg, Y., Glaser, F., Martz, E., Pupko, T., et al. (2005). ConSurf 2005: The projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Research, 33(Web Server issue), W299-302.
- ↑ 2.0 2.1 Borrok, M. J., Kiessling, L. L., & Forest, K. T. (2007). Conformational changes of glucose/galactose-binding protein illuminated by open, unliganded, and ultra-high-resolution ligand-bound structures. Protein Science : A Publication of the Protein Society, 16(6), 1032-1041.