User:Grace Natalie
From Proteopedia
| Line 42: | Line 42: | ||
enhance the ammonium binding, and then to deprotonate the ammonium ion to form the active ammonia | enhance the ammonium binding, and then to deprotonate the ammonium ion to form the active ammonia | ||
to attack the gamma-glutamyl phosphate.</font> | to attack the gamma-glutamyl phosphate.</font> | ||
| - | + | <br><br> | |
| + | <font size=4 face ="Arial">More Catalytic Residues</font> | ||
| + | <br> | ||
| + | <table border=1> | ||
| + | <tr> | ||
| + | <td width=120>Frist</td> | ||
| + | <td width=130>Second</td> | ||
| + | </tr><tr> | ||
| + | <td width=120>Fourth</td> | ||
| + | <td width=130>Fifth</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td width=120>Frist</td> | ||
| + | <td width=130>Second</td> | ||
| + | </tr><tr> | ||
| + | <td width=120>Fourth</td> | ||
| + | <td width=130>Fifth</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td width=120>Frist</td> | ||
| + | <td width=130>Second</td> | ||
| + | </tr><tr> | ||
| + | <td width=120>Fourth</td> | ||
| + | <td width=130>Fifth</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td width=120>Frist</td> | ||
| + | <td width=130>Second</td> | ||
| + | </tr><tr> | ||
| + | <td width=120>Fourth</td> | ||
| + | <td width=130>Fifth</td> | ||
| + | </tr> | ||
| + | </table> | ||
University of Maryland Baltimore County - BioChemistry | University of Maryland Baltimore County - BioChemistry | ||
Revision as of 09:21, 7 December 2008
Goals
- To map the ATP binding site
- Indicate which residues stabilize ATP binding
- Indicate which residues are important for activity and how they contribute to catalysis
Background
Glutamine synthetase (GS) catalyzes the ATP-dependent condensation of ammonia and
glutamate to yield glutamine, ADP, and inorganic phosphate in the presence of divalent cations.
The reaction occurs in two steps with γ-glutamyl phosphate as an intermediate and is used by
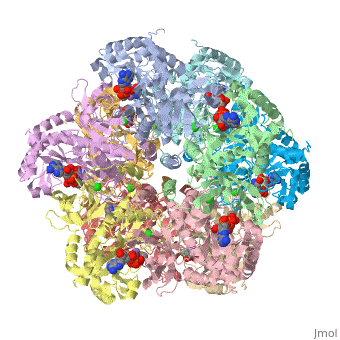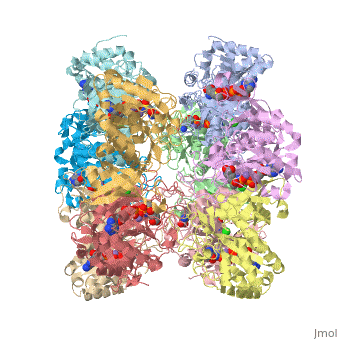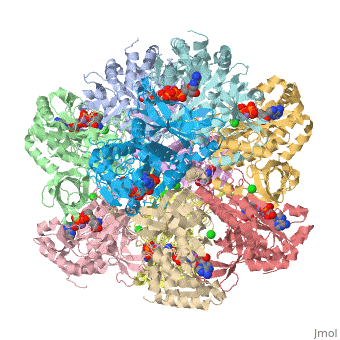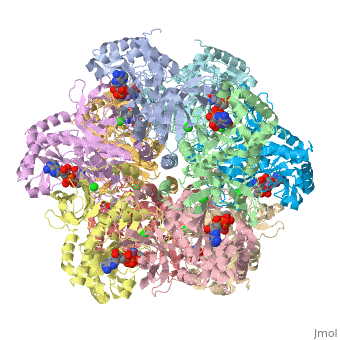
bacteria to introduce reduced nitrogen into cellular metabolism. GS is an enzyme of 12 identical
subunits, arranged in two rings of 6, with the active site between each pair of subunits in a ring.
GS contains two divalent cation sites (n1,n2) and one monovalent cation site per subunit.
Overall Mechanism
ATP first binds to GS, then glutamate binds and attacks ATP to form
gamma-glutamyl phosphate and ADP. An ammounium ion binds to GS and then loses aproton to form the more
active species ammonia, which attacks the gamma-glutamyl phosphate to yield glutamine.
ATP binding site(accompanied by wiki )
ATP binds at the top of the active site cavity and the glutamate binds at the bottom,
adjacent to the n1 ion (Liaw 1994). The movement of Arg 359 toward the glutamate site, induced by
ATP binding, increases the binding affinity of glutamate. The active site of GS is located at the
subunit interface (which contains n1 & n2) and is constituted mainly by the C domain of one subunit
(Liaw 1995).
Involving Residues
Most residues involved in enzymatic catalysis are located at the C domain but Asp50 is
contributed from the N domain of the other subunit. The binding of ADP induces Asp50’ in order to
enhance the ammonium binding, and then to deprotonate the ammonium ion to form the active ammonia
to attack the gamma-glutamyl phosphate.
More Catalytic Residues
| Frist | Second |
| Fourth | Fifth |
| Frist | Second |
| Fourth | Fifth |
| Frist | Second |
| Fourth | Fifth |
| Frist | Second |
| Fourth | Fifth |
University of Maryland Baltimore County - BioChemistry
|