We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Yongmin Kim
From Proteopedia
(Difference between revisions)
| Line 33: | Line 33: | ||
'''Glutamic Amino Acid Binding Site''' | '''Glutamic Amino Acid Binding Site''' | ||
| + | |||
| + | Glutamic amino acid is a product of the hydrolysis of proteins, and it is created by Glutamine in Proteins. It means that glutamine is converted to Glutamic amino acid during the hydrolysis of proteins. In Glutamine Synthetase, Glutamic amino acid binds well with Nitrogen of ATP. | ||
| + | |||
| + | The interaction of main-chain carbonyl oxygen containing Nitrogen in Glutamine Synthetase, is replaced by one with the side-chain of Glu-325 and Ser-154.[8] The amino acid residues Ser-53 and Asn-264 stabilize Glutamic acid in the binding site.[2] | ||
| + | |||
| + | Phosphinothricin resides with glutamate substrate pocket and stabilizes the Glu-327 flap in a site which hinders the access of glutamate into the active site, by holding the inhibitor in the enzyme. | ||
| + | |||
| + | When Glutamic amino acid binds in inhibitor Phosphinothricin between Glu-324 ~ 329, only Glu-327 flap which appears when MetSox or Phosphinothricin binds to Glutamine Synthatase will stabilize the orientation of phosphinyl group by guard the glutamate entrance into the bifunnel. | ||
| + | |||
| + | '''Reference''' | ||
| + | |||
| + | [1] Eisenberg, D., et.al., Structure-function relationships of glutamine synthetases, Biochim Biophys Acta 2000: 1477, 122-145. | ||
| + | [2] Gill, H & Eisenberg, D., Biochemistry 2001 40: 1903-1912 | ||
| + | [3] Krajewski, W. W., et.al.,Structure of Mycobacterium tuberculosis glutamine synthetase in complex with a transition-state mimic provides functional insights, Proc Natl Acad Sci 2005, 102; 10499-10504. | ||
Revision as of 04:09, 8 December 2008
We, Yongmin Kim, Kwangse Lee, and Siwoo Kim, are biology major students at University of Maryland Baltimore Counry, We are enrolled in BIO430 (BioChem), we are planning to complete a project on an aspect of the structure and function of prokaryote glutamine synthetase.
|
Yongmin Kim, Kwangse Lee, Siwoo Kim
Assignment #1
Exercise I
Backbone representation in Rainbow Color
Exercise II
Ligand and Chain selection with Labeling
Exercise III
Active Site Residues
Assignment#2
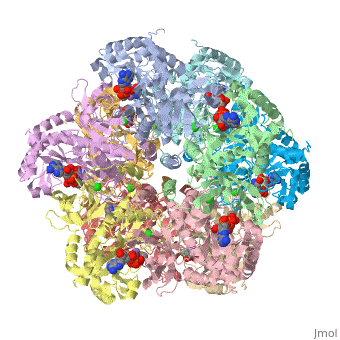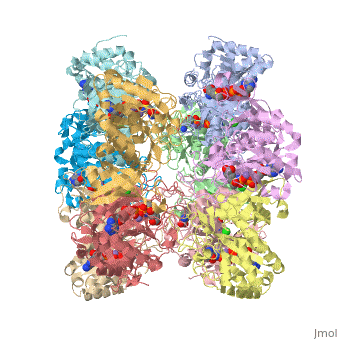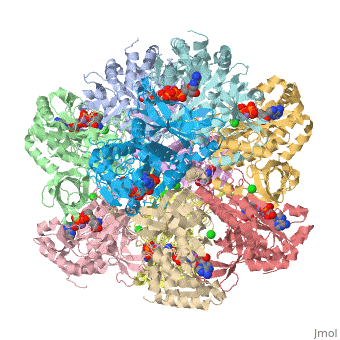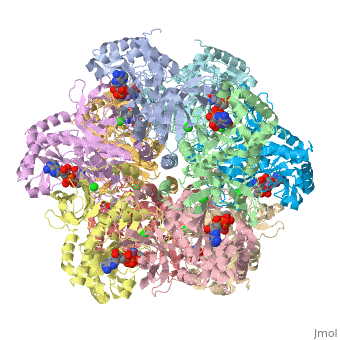
Glutamic Amino Acid Binding Site
Glutamic amino acid is a product of the hydrolysis of proteins, and it is created by Glutamine in Proteins. It means that glutamine is converted to Glutamic amino acid during the hydrolysis of proteins. In Glutamine Synthetase, Glutamic amino acid binds well with Nitrogen of ATP.
The interaction of main-chain carbonyl oxygen containing Nitrogen in Glutamine Synthetase, is replaced by one with the side-chain of Glu-325 and Ser-154.[8] The amino acid residues Ser-53 and Asn-264 stabilize Glutamic acid in the binding site.[2]
Phosphinothricin resides with glutamate substrate pocket and stabilizes the Glu-327 flap in a site which hinders the access of glutamate into the active site, by holding the inhibitor in the enzyme.
When Glutamic amino acid binds in inhibitor Phosphinothricin between Glu-324 ~ 329, only Glu-327 flap which appears when MetSox or Phosphinothricin binds to Glutamine Synthatase will stabilize the orientation of phosphinyl group by guard the glutamate entrance into the bifunnel.
Reference
[1] Eisenberg, D., et.al., Structure-function relationships of glutamine synthetases, Biochim Biophys Acta 2000: 1477, 122-145.
[2] Gill, H & Eisenberg, D., Biochemistry 2001 40: 1903-1912
[3] Krajewski, W. W., et.al.,Structure of Mycobacterium tuberculosis glutamine synthetase in complex with a transition-state mimic provides functional insights, Proc Natl Acad Sci 2005, 102; 10499-10504.