User:Youngsen Jeng
From Proteopedia
| Line 31: | Line 31: | ||
== Outline of the project(revised) == | == Outline of the project(revised) == | ||
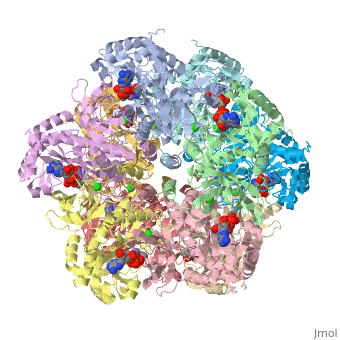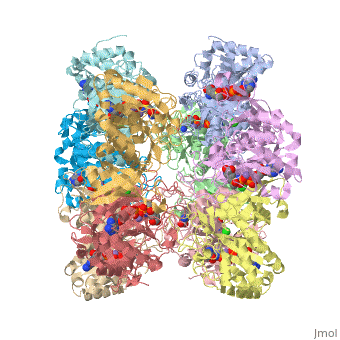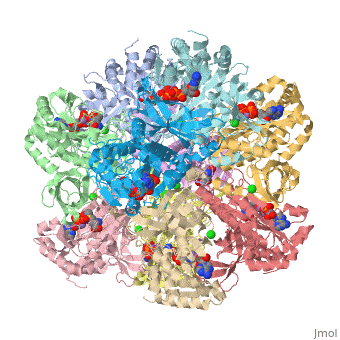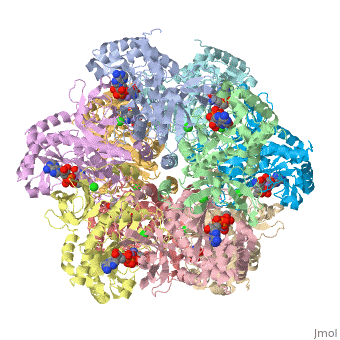
| - | Glutamine synthetase(GS) is an enzyme that plays an essential role in the metabolism of nitrogen by catalyzing the condensation of glutamate and ammonia to form glutamine. Class I enzymes (GSI) are specific to prokaryotes, and are oligomers of 12 identical subunits | + | Glutamine synthetase(GS) is an enzyme that plays an essential role in the metabolism of nitrogen by catalyzing the condensation of glutamate and ammonia to form glutamine. Class I enzymes (GSI) are specific to prokaryotes, and are oligomers of <scene name='User:Youngsen_Jeng/12_identical_subunits/1'>12 identical subunits</scene> The activity of Glutamine Synthetase I-type enzyme is controlled by the <scene name='User:Youngsen_Jeng/Adenylation_of_a_tyrosine/2'>adenylation of tyrosine residue</scene><br> |
The positively charged monovalent cation contributes to the structural stability of GS. Ginsburg and Stadtman(1973) concluded that dodecameric E. coli Glutamine Synthetase is stabilized by Mn2+ and Mg2+<'''Insert wiki here'''>, and that monovalent cations stabilize of the quaternary structure of GS. Stabilization by the monovalent cation may be due both to the electrostatic effects of its positive charge and to other components of the energy of the metal-protein bonding. Interactions of the positively charged monovalent cation with the negatively charged substrate glutamate, Glu 212, Ser 53’, and Asp 50’ could strengthen the active conformation<'''Insert wiki here'''>. Because Ser 53’and Asp 50’reside at the subunit contact surface, the monovalent cation enhances the “side to side” intersubunit interaction. | The positively charged monovalent cation contributes to the structural stability of GS. Ginsburg and Stadtman(1973) concluded that dodecameric E. coli Glutamine Synthetase is stabilized by Mn2+ and Mg2+<'''Insert wiki here'''>, and that monovalent cations stabilize of the quaternary structure of GS. Stabilization by the monovalent cation may be due both to the electrostatic effects of its positive charge and to other components of the energy of the metal-protein bonding. Interactions of the positively charged monovalent cation with the negatively charged substrate glutamate, Glu 212, Ser 53’, and Asp 50’ could strengthen the active conformation<'''Insert wiki here'''>. Because Ser 53’and Asp 50’reside at the subunit contact surface, the monovalent cation enhances the “side to side” intersubunit interaction. | ||
Structural stabilization of Glutamine Synthetase by divalent cations, especially by the n1 ion<'''Insert wiki here'''>, has been ascribed to the attraction of their positive charges to the negative charges of glutamate and ATP and of their ligands<'''Insert wiki here'''> (Liaw et al., 1993~). | Structural stabilization of Glutamine Synthetase by divalent cations, especially by the n1 ion<'''Insert wiki here'''>, has been ascribed to the attraction of their positive charges to the negative charges of glutamate and ATP and of their ligands<'''Insert wiki here'''> (Liaw et al., 1993~). | ||
Revision as of 22:40, 18 December 2008
Youngsen Jeng University of Maryland, Baltimore County Undergraduate Student Biological Science
Partnered with Youngdae Kim for a project. University of Maryland, Baltimore County Undergraduate Student Biological Science
Contents |
Project
| |||||||||
| 2qc8, resolution 2.60Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , , | ||||||||
| Gene: | GLUL, GLNS (Homo sapiens) | ||||||||
| Activity: | Glutamate--ammonia ligase, with EC number 6.3.1.2 | ||||||||
| Related: | 2ojw | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Glutamine Synthetase Exercise 1 by Youngsen&Youngdae
Exercise 1Dr Gluick's class
Glutamine Synthetase Exercise 2 by Youngsen&Youngdae
Exercise 2Dr. Gluick's class
Glutamine Synthetase Exercise 3 by Youngsen&Youngdae
Exercise 3Dr Gluick's class
Glutamine Synthetase Exercise 4 by Youngsen&Youngdae
Exercise 4Dr Gluick's class
Outline of the project(revised)
Glutamine synthetase(GS) is an enzyme that plays an essential role in the metabolism of nitrogen by catalyzing the condensation of glutamate and ammonia to form glutamine. Class I enzymes (GSI) are specific to prokaryotes, and are oligomers of The activity of Glutamine Synthetase I-type enzyme is controlled by the
The positively charged monovalent cation contributes to the structural stability of GS. Ginsburg and Stadtman(1973) concluded that dodecameric E. coli Glutamine Synthetase is stabilized by Mn2+ and Mg2+<Insert wiki here>, and that monovalent cations stabilize of the quaternary structure of GS. Stabilization by the monovalent cation may be due both to the electrostatic effects of its positive charge and to other components of the energy of the metal-protein bonding. Interactions of the positively charged monovalent cation with the negatively charged substrate glutamate, Glu 212, Ser 53’, and Asp 50’ could strengthen the active conformation<Insert wiki here>. Because Ser 53’and Asp 50’reside at the subunit contact surface, the monovalent cation enhances the “side to side” intersubunit interaction.
Structural stabilization of Glutamine Synthetase by divalent cations, especially by the n1 ion<Insert wiki here>, has been ascribed to the attraction of their positive charges to the negative charges of glutamate and ATP and of their ligands<Insert wiki here> (Liaw et al., 1993~).