2bo5
From Proteopedia
OCA (Talk | contribs)
(New page: 200px<br /><applet load="2bo5" size="450" color="white" frame="true" align="right" spinBox="true" caption="2bo5" /> '''BOVINE OLIGOMYCIN SENSITIVITY CONFERRAL PROT...)
Next diff →
Revision as of 06:43, 21 November 2007
|
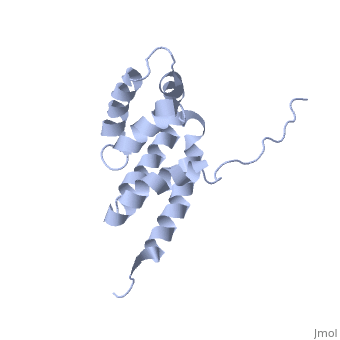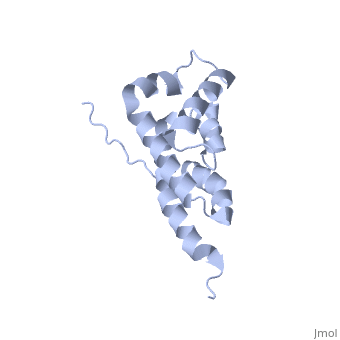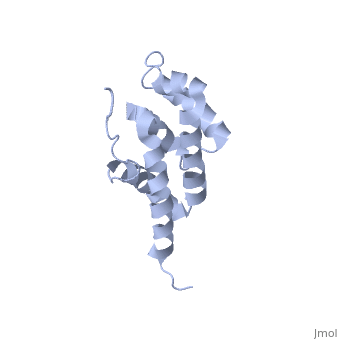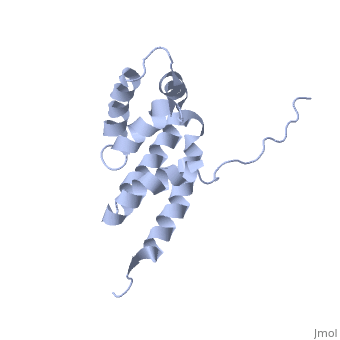
BOVINE OLIGOMYCIN SENSITIVITY CONFERRAL PROTEIN N-TERMINAL DOMAIN
Overview
The peripheral stalk of ATP synthase holds the alpha3beta3 catalytic, subcomplex stationary against the torque of the rotating central stalk. In, bovine mitochondria, the N-terminal domain of the oligomycin sensitivity, conferral protein (OSCP-NT; residues 1-120) anchors one end of the, peripheral stalk to the N-terminal tails of one or more alpha-subunits of, the F1 subcomplex. Here we present the solution structure of OSCP-NT and, an NMR titration study of its interaction with peptides representing, N-terminal tails of F1 alpha-subunits. The structure comprises a bundle of, six alpha-helices, and its interaction site contains adjoining hydrophobic, surfaces of helices 1 and 5; residues in the region 1-8 of the, alpha-subunit are essential for the interaction. The OSCP-NT is similar to, the N-terminal domain of the delta-subunit from Escherichia coli ATP, synthase (delta-NT), except that their surface charges differ (basic and, acidic, respectively). As the charges of the adjacent crown regions in, their alpha3beta3 complexes are similar, the OSCP-NT and delta-NT probably, do not contact the crowns extensively. The N-terminal tails of, alpha-subunit tails are probably alpha-helical, and so this interface, which is essential for the rotary mechanism of the enzyme, appears to, consist of helix-helix interactions.
About this Structure
2BO5 is a Single protein structure of sequence from Bos taurus. Active as H(+)-transporting two-sector ATPase, with EC number 3.6.3.14 Full crystallographic information is available from OCA.
Reference
Structure of the F1-binding domain of the stator of bovine F1Fo-ATPase and how it binds an alpha-subunit., Carbajo RJ, Kellas FA, Runswick MJ, Montgomery MG, Walker JE, Neuhaus D, J Mol Biol. 2005 Aug 26;351(4):824-38. PMID:16045926
Page seeded by OCA on Wed Nov 21 08:51:12 2007
Categories: Bos taurus | H(+)-transporting two-sector ATPase | Single protein | Carbajo, R.J. | Kellas, F.A. | Montgomery, M.G. | Neuhaus, D. | Runswick, M.J. | Walker, J.E. | Alpha-subunit | Atp synthase | Beta-subunit | Binding interface | Cf(1) | Chemical shift mapping | Chemical shift perturbations | Hydrogen ion transport | Hydrolase | Ion transport | Mitochondrion | Nmr | Oscp | Peripheral stalk | Protein-protein interactions | Titration | Transit peptide | Transport