Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox 4
From Proteopedia
| Line 8: | Line 8: | ||
or leave the SCENE parameter empty for the default display. | or leave the SCENE parameter empty for the default display. | ||
--> | --> | ||
| - | {{STRUCTURE_1tsj| PDB=1tsj | SCENE= }} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
=Crystal structure of protein from Staphylococcus aureus= | =Crystal structure of protein from Staphylococcus aureus= | ||
Revision as of 16:53, 18 February 2009
Crystal structure of protein from Staphylococcus aureus
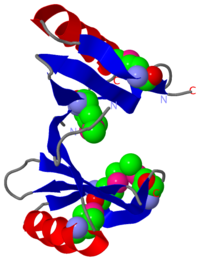
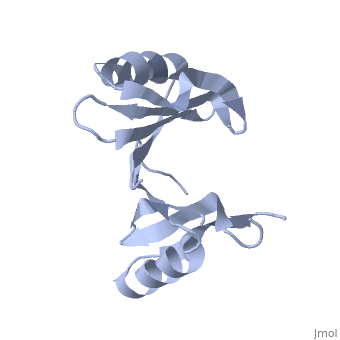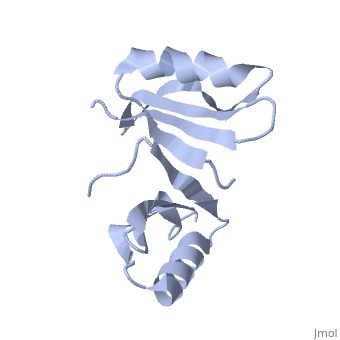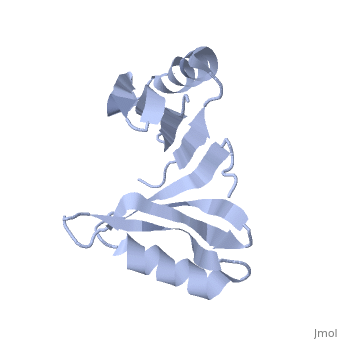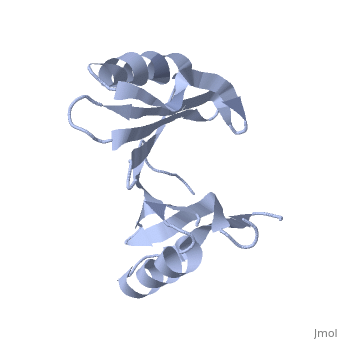
PDB entry 1tsj refers to a hypothetical protein of 139 residues which is a predicted dimer[1] and predicted as a cytoplasmic protein.[2]
The protein is associated with Pfam[3] entry PF06983 of 3-demethylubiquinone-9 3-methyltransferases.
Among the sequence homologs found by PSI-Blast[4] there are the predicted 3-demethylubiquinone-9 3-methyltransferase proteins Q192X9 from Desulfitobacterium hafniense and A9VFW6 from Bacillus weihenstephanensis. Its potential substrate is S-adenosyl-L-methionine with the formal charge +1.
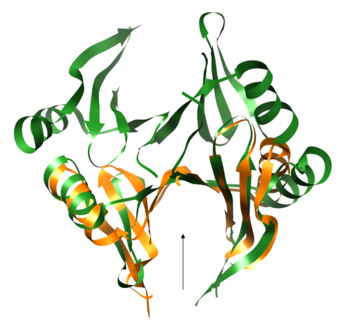
PDB entry 2rk9 was found to be structurally[5] similar to the query protein Q8NX24 and share a significant sequence similarity[6] of 21.0%. This structure homolog is an Oxidoreductase from Vibrio Splendidus and binds methylglyoxal which is not charged. Another structural similarity was found with PDB entry 1t47 which is a 4-hydroxyphenylpyruvate dioxygenase, but the sequence similarity is lower (7.7%).
Both the sequence and structure homologs are from the same superfamily titled 'Glyoxalase/bleomycin resistance protein/dioxygenase'. The Superimposition[7] between chain A of Q8NX24 (orange) and chain B of 2rk9 (green) shows that the proteins indeed have similar folds.
It seems that the chosen active site of Q8NX24 is the largest cavity found on the protein's surface, in most cases this cavity is the functional area.[8] Evidence approving this choice is that the superimpositions with 2rk9 placed its known active site[9] at the same location.
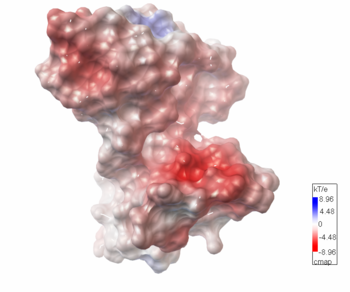
The electrostatic potential[10] on the protein's surface is mostly negative.
|

The potential substrate S-adenosyl-L-methionine has positive formal charge which fits the negative electrostatic potential in the potential catalytic area. The alternative substrate found by the structural homology methylglyoxal is not charged and thus less probable to attach to the potential catalytic area.
It seems more likely that Q8NX24 belongs to the Glyoxalase/bleomycin resistance protein/dioxygenase superfamily and functions as a demethylubiquinone-9 3-methyltransferase protein.
References
- ↑ Henrick, K., and Thornton, J.M. (1998). PQS: a protein quaternary structure file server. Trends Biochem. Sci. 23, 358- 361.
- ↑ Bhasin, M., Garg, A. and Raghava, GPS (2005) PSLpred: prediction of subcellular localization of bacterial proteins. Bioinformatics. 21, 2522-2524. Gardy JL, Spencer C, Wang K, Ester M, Tusn?dy GE, Simon I, Hua S, deFays K, Lambert C, Nakai K, Brinkman FS. (2003) PSORT-B: improving protein subcellular localization prediction for Gram-negative bacteria. Nucleic Acids Res. 31, 3613-3617. Z. Lu, D. Szafron, R. Greiner, P. Lu, D.S. Wishart, B. Poulin, J. Anvik, C. Macdonell, and R. Eisner. (2003). Predicting Subcellular Localization of Proteins using Machine-Learned Classifiers. Bioinformatics. 20, 547-556. Hua S, Sun Z. (2001). Support vector machine approach for protein subcellular localization prediction. Bioinformatics. 17, 721-728.
- ↑ Finn R, Griffiths-Jones S, Bateman A. (2003). Identifying protein domains with the Pfam database. Curr Protoc Bioinformatics. Chapter 2: Unit 2.5.
- ↑ Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389-3402.
- ↑ Arnold K, Kiefer F, Kopp J, Battey JN, Podvinec M, Westbrook JD, Berman HM, Bordoli L, Schwede T. (2008). The Protein Model Portal. J Struct Funct Genomics.
- ↑ Emmert D.B., Stoehr P.J., Stoesser G., Cameron G.N. (1994). The European Bioinformatics Institute (EBI) databases. Nucleic Acids Res. 26, 3445-3449.
- ↑ Pettersen, E.F., Goddard, T.D., Huang, C.C., Couch, G.S., Greenblatt, D.M., Meng, E.C., and Ferrin, T.E. (2004). UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605-1612.
- ↑ J. Liang, H. Edelsbrunner, and C. Woodward, (1998). Anatomy of protein pockets and cavities: measurement of binding site geometry and implications for ligand design. Protein Sci. 7, 1884–1897.
- ↑ Thornalley P.J., (2003). Glyoxalase I – structure, function and a critical role in the enzymatic defence against glycation. Biochemical Soc Trans. 31, 1343-1348.
- ↑ Sanner, M.F. (1999). Python: a programming language for software integration and development. J. Mol. Graph. Model. 17, 57-61.
- ↑ Goldenberg O, Erez E, Nimrod G, Ben-Tal N. (2009). The ConSurf-DB: pre-calculated evolutionary conservation profiles of protein structures. Nucleic Acids Res. 37, D323-327.
Categories: Staphylococcus aureus subsp. aureus | Burley, S K. | Gorman, J. | Min, T. | NYSGXRC, New York Structural GenomiX Research Consortium. | Shapiro, L. | Conserved hypothetical protein | Crystal structure | New york structural genomics consortium | New york structural genomix research consortium | Nysgxrc | Protein structure initiative | Psi | Structural genomic