User:Maitreyee Mukherjee/Sandbox 1
From Proteopedia
| Line 116: | Line 116: | ||
<font color='red'>'''HOW WAS THE ANIMATED IMAGE GENERATED?'''</font> | <font color='red'>'''HOW WAS THE ANIMATED IMAGE GENERATED?'''</font> | ||
| - | 1. Go to the POLYVIEW 3D homepage, http://polyview.cchmc.org/polyview3d.html | + | 1. Go to the POLYVIEW 3D homepage, http://polyview.cchmc.org/polyview3d.html |
2. On the submission form, first select 'animation' in the "type of request" section, select the size of the animation to be genrated in pixels, then upload the PDB format protein structure file in the "source of structural data" section. | 2. On the submission form, first select 'animation' in the "type of request" section, select the size of the animation to be genrated in pixels, then upload the PDB format protein structure file in the "source of structural data" section. | ||
Revision as of 17:35, 26 April 2009
|
INTRODUCTION:
Rhodobacter sphaeroides is a metabolically versatile purple photosynthetic α-proteobacteria which produces polyhydroxybutyrate (PHB) as inclusions inside its cell. PHB is a biopolymer which has the properties similar to synthetic polymers such as polyethylene and hence has a potential of being used as a biopolymer. The biochemical pathway for PHB production in this organism is complex and is controlled by the actions of several gene products. PHB synthase encoded by phaC is responsible for conversion of the monomeric precursor of PHB R(-)-β-Hydroxybutyryl-CoA into polyhydroxybutyrate.Rhodobacter sphaeroides possesses the Class I phylogenetic group of PHA synthases. Class I PHA synthases prefer utilization of CoA thioesters of various (R)-3-hydroxy fatty acids comprising of 3 to 5 carbon atoms.
FUNCTION:
The enzyme catalyses the conversion of the monomeric precursor of PHB into PHB with the release of CoA. In other words these enzymes mediate the conversion of a soluble substrate into polymerized insoluble inclusions inside the cells of these bacteria. Upon covalent catalysis of polyester chain formation, this soluble enzyme gets converted to amphipathic enzyme (The amphipathic enzyme conjugates are analogous to certain membrane-bound proteins such as cytochrome b, which have been shown to comprise a hydrophilic protein core anchored to a lipid bilayer by a hydrophobic polypeptide tail). Initiation of a self assembly process occurs and this results in the formation of the insoluble cytoplasmic inclusions with a phospholipids monolayer and covalently attached polyester synthases at the surface.
PROPERTIES:
Number of amino acids: 601
Molecular weight: 66831.6
Theoretical pI: 5.63
Amino acid sequence:
1 mateeqspgs grdaqferln anltridels krltaaltkr klsdpalhgp sgdvflkamt
61 aymaemmqnp akilehqisf wgkslkhyve aqhqlvkgel kpppdvtpkd rrfsnplwqt
121 hpffnylkqq ylmnaeavnq avealehiep sdkkrveyfs rqivdlfspt nffgtnpdal
181 eraiatdges lvqglenlvr dieanngdll vtladpeafq vgqnlatteg svvyrnrmfe
241 liqykpttet vhetpllifp pwinkfyild lkpqnsllkw lvdqgftvfv vswvnpdksy
301 agigmddyir egymramaev rsitrqkqin avgyciagtt ltltlahlqk agdpsvrsat
361 ffttltdfsd pgevgvflnd dfvdgierqv avdgildktf msrtfsylrs ndliyqpaik
421 symmgeappa fdllywngdg tnlpaqmave ylrglcqqdr laggtfpvlg spvglkdvtl
481 pvcaiacetd hiapwkssfn gfrqfgstdk tfilsqsghv agivnppsrn kyghytnegp
541 agtpesfreg aefhagswwp rwgawlaers gkqvparqpg dskhpelapa pgsyvaavgg
601 a
Amino acid Composition:
Ala (A) 52 8.7%, Arg (R) 29 4.8%, Asn (N) 26 4.3%, Asp (D) 34 5.7%, Cys (C) 4 0.7%, Gln (Q) 29 4.8%, Glu (E) 35 5.8%, Gly (G) 46 7.7%, His (H) 13 2.2%, Ile (I) 24 4.0%, Leu (L) 54 9.0%, Lys (K) 28 4.7%, Met (M) 14 2.3%, Phe (F) 31 5.2%, Pro (P) 40 6.7%, Ser (S) 36 6.0%, Thr (T) 37 6.2%, Trp (W) 11 1.8%, Tyr (Y) 20 3.3%. Val (V) 38 6.3% Pyl (O) 0 0.0% Sec (U) 0 0.0%
Total number of negatively charged residues (Asp + Glu): 69 Total number of positively charged residues (Arg + Lys): 57
Atomic composition:
Carbon C 3004 Hydrogen H 4612 Nitrogen N 808 Oxygen O 888 Sulfur S 18
Total number of atoms: 9330
Extinction coefficients:
Extinction coefficients are in units of M-1 cm-1, at 280 nm measured in water.
Ext. coefficient 90550 Abs 0.1% (=1 g/l) 1.355, assuming ALL Cys residues appear as half cystines Ext. coefficient 90300 Abs 0.1% (=1 g/l) 1.351, assuming NO Cys residues appear as half cystines
Estimated half-life:
The N-terminal of the sequence considered is M (Met).
The estimated half-life is: 30 hours (mammalian reticulocytes, in vitro). >20 hours (yeast, in vivo). >10 hours (Escherichia coli, in vivo).
Instability index:
The instability index (II) is 41.97 This classifies the protein as unstable.
Aliphatic index: 77.60
Grand average of hydropathicity (GRAVY): -0.330
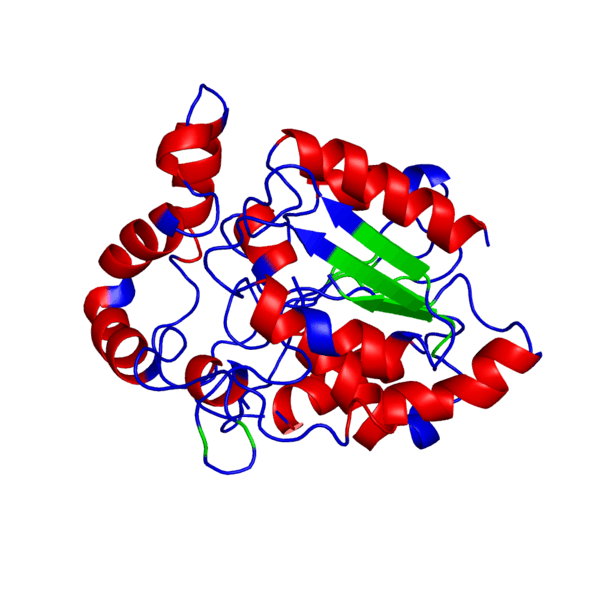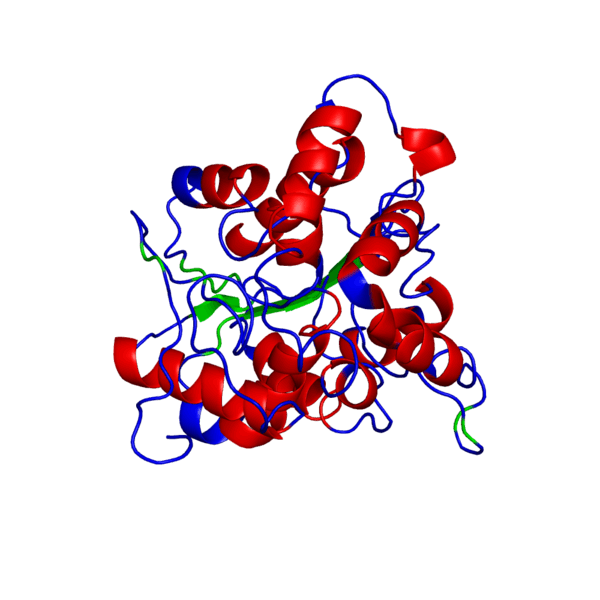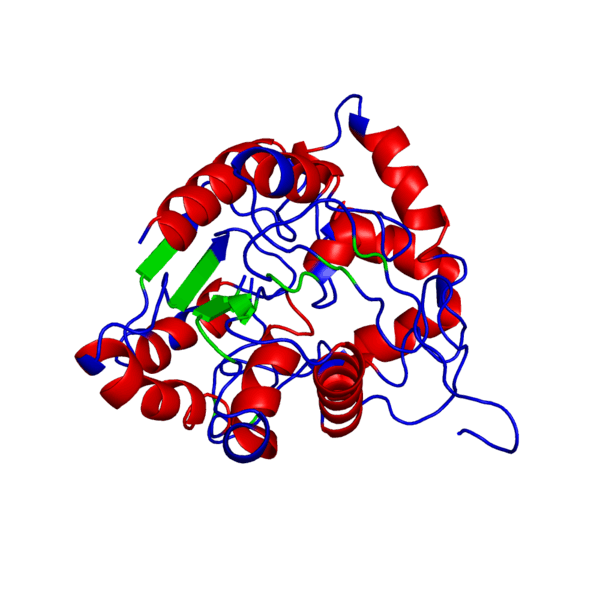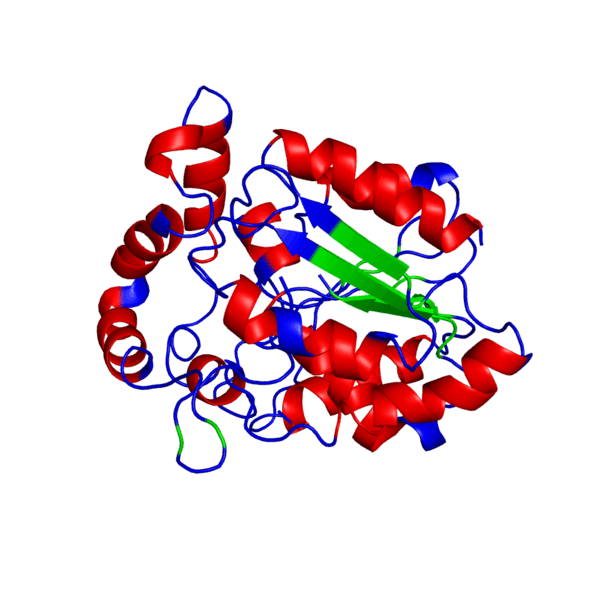
ANIMATED IMAGE OF THE PUTATIVE PHB SYNTHASE DOMAIN SHOWING AMINO ACIDS 286 TO 547 IN Rhodobacter sphaeroides OBTAINED FROM POLYVIEW-3D
HOW WAS THE ANIMATED IMAGE GENERATED?
1. Go to the POLYVIEW 3D homepage, http://polyview.cchmc.org/polyview3d.html
2. On the submission form, first select 'animation' in the "type of request" section, select the size of the animation to be genrated in pixels, then upload the PDB format protein structure file in the "source of structural data" section.
3. On the "chain color and rendering section" select 'cartoon' and 'secondary structure'.
4. On "advanced structural annotation" section select 'docking models in Capri format'.
5. Any other forms for the animation may be selected by referring to the "Samples" according to the protein structure to be animated.
HOW WAS THE JMOL IMAGE GENERATED?
REFERENCES:
1. Fales, L., L. Kryszak., and J. Zeilstra-Ryalls. 2001. Control of hemA expression in Rhodobacter sphaeroides 2.4.1: effect of a transposon insertion in the hbdA gene. Journal of Bacteriology. 183:1568–1576."PMID:"11160087
2. Hustede, E., and A. Steinbuchel. 1993. Characterization of the polyhydroxyalkanoate synthase gene locus of Rhodobacter sphaeroides. Biotechnology Letters. 15:709–714.
3. Kolibachuk, D., A. Miller., and D. Dennis. 1999. Cloning, molecular analysis, and expression of the polyhydroxyalkanoic acid synthase (phaC) gene from Chromobacterium violaceum. Applied and Environmental Microbiology. 65:3561–3565.
4. Kranz, R.G., K. K. Gabbert., T. A. Locke., and M. T. Madigan. 1997. Polyhydroxyalkanoate production in Rhodobacter capsulatus: genes, mutants, expression, and physiology. Applied and Environmental Microbiology. 63:3003–3009.
5. Peoples, P.O., and A. J. Sinskey. 1989. Poly-ß-hydroxybutyrate (PHB) biosynthesis in Alcaligenes eutrophus H16, identification and characterization of the PHB polymerase gene (phbC). The Journal of Biological Chemistry. 264(26):15298-15303.
6. Rehm, H. A. B., and A. Steinbüchel. 1999. Biochemical and genetic analysis of PHA synthases and other proteins required for PHA synthesis. International Journal of Biological Macromolecules. 25:3-19.
7. Rehm, Bernd H. A. B. 2003. Polyester synthases: natural catalysts for plastics. Biochemical Journal. 376:15–33.
8. Stubbe, J. A., and J. Tian. 2003. Polyhydroxyalkanoate (PHA) homeostasis: the role of PHA synthase. Natural Product Reports. 20:445-457.
9. Tae-Kwon, K. J. Young-Mi. M. Tri Vo., S.Suteaki., and L.Yong-Hyun. 2006. Metabolic engineering and characterization of phaC1 and phaC2 genes from Pseudomonas putida KCTC1639 for overproduction of medium-chain-length polyhydroxyalkanoate. Biotechnology Progress.22: 1541-1546.
10. Ueda, S., T. Yabutani., A. Maehara., and T. Yamane. 1996. Molecular analysis of the poly(3-hydroxyalkanoate) synthase gene from a methylotrophic bacterium, Paracoccus denitrificans. Journal of Bacteriology. 178:774–779.