User:Maitreyee Mukherjee/Sandbox 1
From Proteopedia
| Line 1: | Line 1: | ||
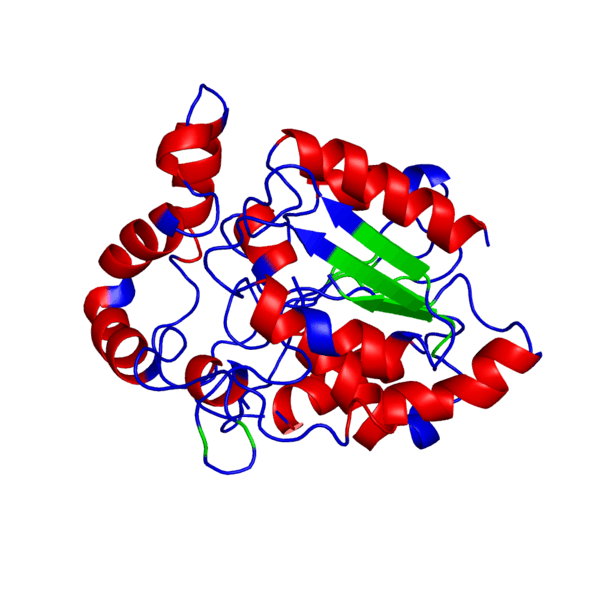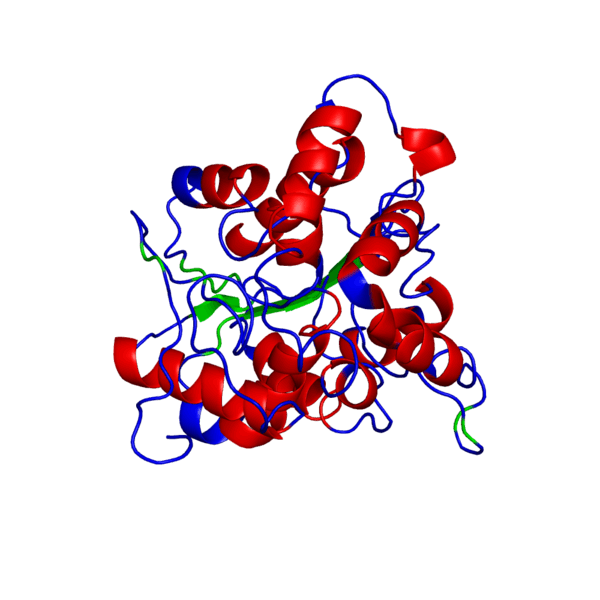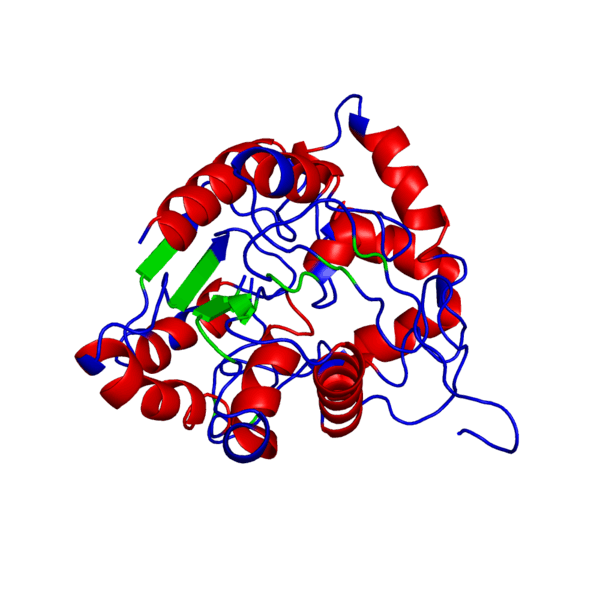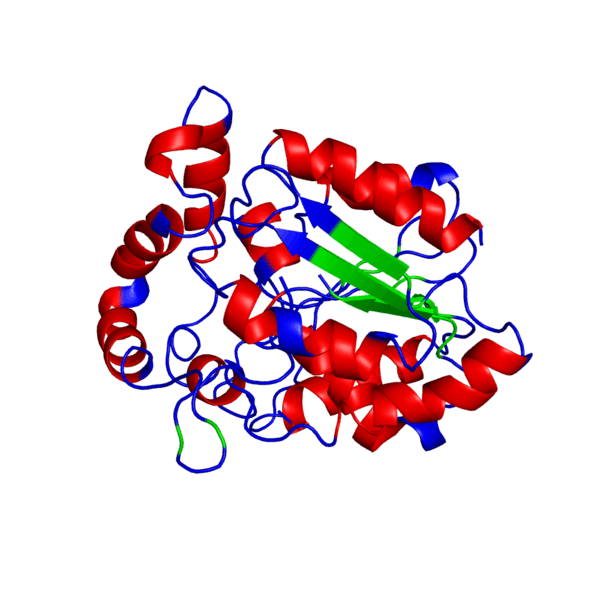
<applet load='FILE_consurf1240241051_pipe.pdb' size='500' frame='true' align='right' caption='Putative PHB synthase domain in Rhodobacter sphaeroides,amino acids 286 to 547' /> | <applet load='FILE_consurf1240241051_pipe.pdb' size='500' frame='true' align='right' caption='Putative PHB synthase domain in Rhodobacter sphaeroides,amino acids 286 to 547' /> | ||
| - | + | =Introduction= | |
| - | ''Rhodobacter sphaeroides'' is a metabolically versatile purple photosynthetic α-proteobacteria<ref>PMID: 14999403</ref> which produces polyhydroxybutyrate (PHB) as inclusions inside its cell. PHB is a biopolymer which has the properties similar to synthetic polymers such as polyethylene and hence has a potential of being used as a biopolymer. The biochemical pathway for PHB production in this organism is complex and is controlled by the actions of several gene products<ref>PMID:10416645</ref>. PHB synthase encoded by ''phaC'' is responsible for conversion of the monomeric precursor of PHB R(-)-β-Hydroxybutyryl-CoA into polyhydroxybutyrate <ref> Hustede, E., and A. Steinbuchel. 1993. Characterization of the polyhydroxyalkanoate synthase gene locus of Rhodobacter sphaeroides. Biotechnology Letters. 15:709–714.</ref>.''Rhodobacter sphaeroides'' possesses the Class I phylogenetic group of PHA synthases<ref>PMID:14620841</ref>. Class I PHA synthases prefer utilization of CoA thioesters of various (R)-3-hydroxy fatty acids comprising of 3 to 5 carbon atoms<ref>PMID:12954080</ref>. | + | ''Rhodobacter sphaeroides'' is a metabolically versatile purple photosynthetic α-proteobacteria<ref>PMID: 14999403</ref> which produces polyhydroxybutyrate (PHB) as inclusions inside its cell. PHB is a biopolymer which has the properties similar to synthetic polymers such as polyethylene and hence has a potential of being used as a biopolymer. The biochemical pathway for PHB production in this organism is complex and is controlled by the actions of several gene products<ref>PMID:10416645</ref>. PHB synthase encoded by ''phaC'' is responsible for conversion of the monomeric precursor of PHB R(-)-β-Hydroxybutyryl-CoA into polyhydroxybutyrate <ref> Hustede, E., and A. Steinbuchel. 1993. Characterization of the polyhydroxyalkanoate synthase gene locus of Rhodobacter sphaeroides. Biotechnology Letters. 15:709–714.</ref>.''Rhodobacter sphaeroides'' possesses the Class I phylogenetic group of PHA synthases<ref>PMID:14620841</ref>. Class I PHA synthases prefer utilization of CoA thioesters of various (R)-3-hydroxy fatty acids comprising of 3 to 5 carbon atoms<ref>PMID:12954080</ref>. |
| - | + | =Function= | |
The enzyme catalyses the conversion of the monomeric precursor of PHB into PHB with the release of CoA. In other words these enzymes mediate the conversion of a soluble substrate into polymerized insoluble inclusions inside the cells of these bacteria. | The enzyme catalyses the conversion of the monomeric precursor of PHB into PHB with the release of CoA. In other words these enzymes mediate the conversion of a soluble substrate into polymerized insoluble inclusions inside the cells of these bacteria. | ||
Revision as of 15:57, 27 April 2009
|
Introduction
Rhodobacter sphaeroides is a metabolically versatile purple photosynthetic α-proteobacteria[1] which produces polyhydroxybutyrate (PHB) as inclusions inside its cell. PHB is a biopolymer which has the properties similar to synthetic polymers such as polyethylene and hence has a potential of being used as a biopolymer. The biochemical pathway for PHB production in this organism is complex and is controlled by the actions of several gene products[2]. PHB synthase encoded by phaC is responsible for conversion of the monomeric precursor of PHB R(-)-β-Hydroxybutyryl-CoA into polyhydroxybutyrate [3].Rhodobacter sphaeroides possesses the Class I phylogenetic group of PHA synthases[4]. Class I PHA synthases prefer utilization of CoA thioesters of various (R)-3-hydroxy fatty acids comprising of 3 to 5 carbon atoms[5].
Function
The enzyme catalyses the conversion of the monomeric precursor of PHB into PHB with the release of CoA. In other words these enzymes mediate the conversion of a soluble substrate into polymerized insoluble inclusions inside the cells of these bacteria.
PROPERTIES:
| N | C |
| Amino Terminus | Carboxy Terminus |
| 5' | 3' |
| N, 5' | C, 3' |
Hydrophobic, Polar
Alpha Helices, Beta Strands , Turns.
Number of amino acids: 601
Molecular weight: 66831.6
Theoretical pI: 5.63
Amino acid sequence:
1 mateeqspgs grdaqferln anltridels krltaaltkr klsdpalhgp sgdvflkamt
61 aymaemmqnp akilehqisf wgkslkhyve aqhqlvkgel kpppdvtpkd rrfsnplwqt
121 hpffnylkqq ylmnaeavnq avealehiep sdkkrveyfs rqivdlfspt nffgtnpdal
181 eraiatdges lvqglenlvr dieanngdll vtladpeafq vgqnlatteg svvyrnrmfe
241 liqykpttet vhetpllifp pwinkfyild lkpqnsllkw lvdqgftvfv vswvnpdksy
301 agigmddyir egymramaev rsitrqkqin avgyciagtt ltltlahlqk agdpsvrsat
361 ffttltdfsd pgevgvflnd dfvdgierqv avdgildktf msrtfsylrs ndliyqpaik
421 symmgeappa fdllywngdg tnlpaqmave ylrglcqqdr laggtfpvlg spvglkdvtl
481 pvcaiacetd hiapwkssfn gfrqfgstdk tfilsqsghv agivnppsrn kyghytnegp
541 agtpesfreg aefhagswwp rwgawlaers gkqvparqpg dskhpelapa pgsyvaavgg
601 a
Amino acid Composition:
Ala (A) 52 8.7%, Arg (R) 29 4.8%, Asn (N) 26 4.3%, Asp (D) 34 5.7%, Cys (C) 4 0.7%, Gln (Q) 29 4.8%, Glu (E) 35 5.8%, Gly (G) 46 7.7%, His (H) 13 2.2%, Ile (I) 24 4.0%, Leu (L) 54 9.0%, Lys (K) 28 4.7%, Met (M) 14 2.3%, Phe (F) 31 5.2%, Pro (P) 40 6.7%, Ser (S) 36 6.0%, Thr (T) 37 6.2%, Trp (W) 11 1.8%, Tyr (Y) 20 3.3%. Val (V) 38 6.3% Pyl (O) 0 0.0% Sec (U) 0 0.0%
Total number of negatively charged residues (Asp + Glu): 69 Total number of positively charged residues (Arg + Lys): 57
Atomic composition:
Carbon C 3004 Hydrogen H 4612 Nitrogen N 808 Oxygen O 888 Sulfur S 18
Total number of atoms: 9330
Extinction coefficients:
Extinction coefficients are in units of M-1 cm-1, at 280 nm measured in water.
Ext. coefficient 90550 Abs 0.1% (=1 g/l) 1.355, assuming ALL Cys residues appear as half cystines Ext. coefficient 90300 Abs 0.1% (=1 g/l) 1.351, assuming NO Cys residues appear as half cystines
Estimated half-life:
The N-terminal of the sequence considered is M (Met).
The estimated half-life is: 30 hours (mammalian reticulocytes, in vitro). >20 hours (yeast, in vivo). >10 hours (Escherichia coli, in vivo).
Instability index:
The instability index (II) is 41.97 This classifies the protein as unstable.
Aliphatic index: 77.60
Grand average of hydropathicity (GRAVY): -0.330
ANIMATED IMAGE OF THE PUTATIVE PHB SYNTHASE DOMAIN SHOWING AMINO ACIDS 286 TO 547 IN Rhodobacter sphaeroides OBTAINED FROM POLYVIEW-3D
CURRENT RESEARCH: KNOWLEDGE OF THIS PROTEIN IN R.sphaeroides AS WELL AS IN OTHER ORGANISMS:
1. In R.sphaeroides FJ1, phbC has been found to be present on one of two gene locus identified to be involved in the production of PHB. The gene phbC is present on the same locus along with the other genes responsible for PHB syn thesis such as phbZ, phbP and phbR. [6]
2. In a paper by Fales et.al. in 2001, the investigators talk about a possible connection between PHB synthase activity and hemA transcription in R.sphaeroides. No further investigations answering this have been published after this untill now. The investigators also present a schematic diagram of a proposed dual pathway of PHB synthesis in this organism.[7]
3. In Rhodobacter capsulatus, phaC was found to have a constitutive expression in presence or absence of the induction of PHA production, indicating that the control of expression of this gene occurs at the post-translational level in this organism. [8]
4. In Paracoccus denitrificans, an organism having a close similarity in genome to R.sphaeroides, the phaC transcription has been found to be reduced under nitrogen deficient condition, although the mechanism of regulation of this gene is not very clear yet..[9]. in the cited paper, the investigators have included a proposed model for regulation of PHB synthesis in this organism.
5. One group of investigators suggest a role of the genes involved in the nitrogen-related phosphotransferase system (PTS)in PHB accumulation in the organism Azotobacter vinelandii. This group of researchers report that one of the proteins of PTS affect the transcription of the phbBAC operon(consisting of the phbA, phbB and phbC genes) in this organism. Mechanism of this regulation is still unclear.[10]
PROBABLE RESEARCH:FOR BETTER UNDERSTANDING OF THIS PROTEIN:CHALLENGE YOUR UNDERSTANDING
1. What is the molecular basis of regulation of PHB synthesis in this organism and what is the role of this protein in it?
2. How does environmental factors such as presence or absence of oxygen or nitrogen relate to the activity of this protein in R.sphaeroides?
3. What are the activators or repressor factors, if any, responsible for production of this protein in this organism?
4. Is there a possible role of the nitrogen-related Phosphotransferase system in the regulation of expression of PHB synthase in R.sphaeroides?
HOW WAS THE ANIMATED IMAGE GENERATED?
1. Go to the POLYVIEW 3D homepage, http://polyview.cchmc.org/polyview3d.html
2. On the submission form, first select 'animation' in the "type of request" section, select the size of the animation to be generated in pixels(here the size is 600), then upload the PDB format protein structure file in the "source of structural data" section.
3. On the "chain color and rendering section" select 'cartoon' and 'secondary structure'.
4. On "advanced structural annotation" section select 'docking models in Capri format'.
5. Any other forms for the animation may be selected by referring to the "Samples" according to the protein structure to be animated.
HOW WAS THE JMOL IMAGE GENERATED?
1. First retrieve your protein sequence from http://www.ncbi.nlm.nih.gov/.
2. Go to 3D-JIGSAW page http://bmm.cancerresearchuk.org/~3djigsaw/ and paste the sequence on the submission page. A .pdb format image of your protein will be sent to you on your email which can be opened by RASMOL.
3. Upload this file on Proteopedia and then load the JMol applet for the protein following instructions on the Help:Editing page http://www.proteopedia.org/wiki/index.php/Help:Editing.
4. You can edit your protein by using the scene authoring tools after loading the applet.
REFERENCES:
- ↑ Zeilstra-Ryalls JH, Kaplan S. Oxygen intervention in the regulation of gene expression: the photosynthetic bacterial paradigm. Cell Mol Life Sci. 2004 Feb;61(4):417-36. PMID:14999403 doi:10.1007/s00018-003-3242-1
- ↑ Rehm BH, Steinbuchel A. Biochemical and genetic analysis of PHA synthases and other proteins required for PHA synthesis. Int J Biol Macromol. 1999 Jun-Jul;25(1-3):3-19. PMID:10416645
- ↑ Hustede, E., and A. Steinbuchel. 1993. Characterization of the polyhydroxyalkanoate synthase gene locus of Rhodobacter sphaeroides. Biotechnology Letters. 15:709–714.
- ↑ Stubbe J, Tian J. Polyhydroxyalkanoate (PHA) hemeostasis: the role of PHA synthase. Nat Prod Rep. 2003 Oct;20(5):445-57. PMID:14620841
- ↑ Rehm BH. Polyester synthases: natural catalysts for plastics. Biochem J. 2003 Nov 15;376(Pt 1):15-33. PMID:12954080 doi:http://dx.doi.org/10.1042/BJ20031254
- ↑ Yang MK, Lin YC, Shen CH. Identification of two gene loci involved in poly-beta-hydroxybutyrate production in Rhodobacter sphaeroides FJ1. J Microbiol Immunol Infect. 2006 Feb;39(1):18-27. PMID:16440119
- ↑ Fales L, Kryszak L, Zeilstra-Ryalls J. Control of hemA expression in Rhodobacter sphaeroides 2.4.1: effect of a transposon insertion in the hbdA gene. J Bacteriol. 2001 Mar;183(5):1568-76. PMID:11160087 doi:10.1128/JB.183.5.1568-1576.2001
- ↑ Kranz RG, Gabbert KK, Locke TA, Madigan MT. Polyhydroxyalkanoate production in Rhodobacter capsulatus: genes, mutants, expression, and physiology. Appl Environ Microbiol. 1997 Aug;63(8):3003-9. PMID:9251189
- ↑ Kojima T, Nishiyama T, Maehara A, Ueda S, Nakano H, Yamane T. Expression profiles of polyhydroxyalkanoate synthesis-related genes in Paracoccus denitrificans. J Biosci Bioeng. 2004;97(1):45-53. PMID:16233588 doi:http://dx.doi.org/S1389-1723(04)70164-4
- ↑ Noguez R, Segura D, Moreno S, Hernandez A, Juarez K, Espin G. Enzyme I NPr, NPr and IIA Ntr are involved in regulation of the poly-beta-hydroxybutyrate biosynthetic genes in Azotobacter vinelandii. J Mol Microbiol Biotechnol. 2008;15(4):244-54. Epub 2007 Sep 20. PMID:17878711 doi:http://dx.doi.org/000108658
PAGE CREATED BY MAITREYEE MUKHERJEE, email:maitreyee25@gmail.com
ACKNOWLEDGEMENT: Jill Zeilstra Ryalls, Professor, Bowling Green State University.