X-ray crystallography
From Proteopedia
(Difference between revisions)
(→See Also - adding content) |
(→See Also - adding content) |
||
| Line 13: | Line 13: | ||
*[http://www.pdb.org/pdb/static.do?p=general_information\about_pdb\nature_of_3d_structural_data.html Nature of 3D Structural Data] | *[http://www.pdb.org/pdb/static.do?p=general_information\about_pdb\nature_of_3d_structural_data.html Nature of 3D Structural Data] | ||
*[http://en.wikipedia.org/wiki/X-ray_crystallography X-ray Crystallography at Wikipedia] | *[http://en.wikipedia.org/wiki/X-ray_crystallography X-ray Crystallography at Wikipedia] | ||
| + | *[http://hamptonresearch.com/gallery.aspx Protein Crystal Gallery] | ||
*[[Quality assessment for molecular models]] | *[[Quality assessment for molecular models]] | ||
| Line 18: | Line 19: | ||
*[[R value]] | *[[R value]] | ||
*[[Free R]] | *[[Free R]] | ||
| + | |||
*[[Highest impact structures]] of all time. | *[[Highest impact structures]] of all time. | ||
Revision as of 00:24, 19 May 2009
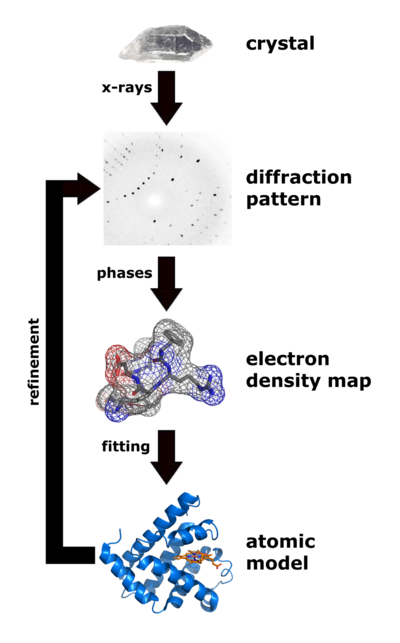
|
| Flow chart showing the major steps in X-ray protein crystallography. (Image from Wikimedia courtesy Thomas Splettstoesser. |
About 85% of the models (entries) in the World Wide Protein Data Bank were determined by X-ray crystallography. (Most of the remaining 15% were determined by solution nuclear magnetic resonance.) Protein crystallography remains very difficult, despite many recent advances. For every new protein sequence targeted for X-ray crystallography, about one in twenty is solved[1][2]. Publication of solved structures involves depositing an atomic coordinate file (PDB file) in the World Wide Protein Data Bank.
See Also
Further Reading
- Crystallography Made Crystal Clear: a guide for users of macromolecular models, a book by Gale Rhodes.
