User:Ramiro Barrantes/FpgNeiRepair
From Proteopedia
(Difference between revisions)
(→Functional Cluster 1: Stability of perfectly conserved Asn168) |
(→Functional Cluster 1: Stability of perfectly conserved Asn168) |
||
| Line 27: | Line 27: | ||
=== Functional Cluster 1: Stability of perfectly conserved Asn168 === | === Functional Cluster 1: Stability of perfectly conserved Asn168 === | ||
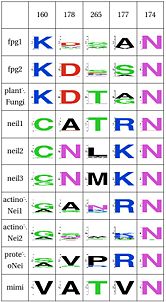
| - | [[Image:F1WebLogo.jpg]] Asn168, along with two other amino acids have an effect in the orientation and kinking of the DNA. In 4 of the 9 clades (Fpg1, Fpg2 and Plants and Fungi) Asn168 is supported by Lys160, which in turn hydrogen bonds with Leu249 and Ser250. In the other clades (Actinobacteria 1 and 2, Proteacteria and all vertebrate sequences), Arg171 that comes from a different helix fulfills the same roles as Lys160. One important difference is that the Zinc Finger is shaped differently in the absence of DNA, and there is a hydrogen bond between one of the | + | [[Image:F1WebLogo.jpg|thumb|166px|left|WebLogo for the first functional unit, note the covariation between lysine nad arginine]] Asn168, along with two other amino acids have an effect in the orientation and kinking of the DNA. In 4 of the 9 clades (Fpg1, Fpg2 and Plants and Fungi) Asn168 is supported by Lys160, which in turn hydrogen bonds with Leu249 and Ser250. In the other clades (Actinobacteria 1 and 2, Proteacteria and all vertebrate sequences), Arg171 that comes from a different helix fulfills the same roles as Lys160. One important difference is that the Zinc Finger is shaped differently in the absence of DNA, and there is a hydrogen bond between one of the beta-sheets and the arginine. One hypothesis is that the arginine or the lysine is necessary to support the Asn168, crucial for orientation of the DNA. |
== Evolution == | == Evolution == | ||
== References == | == References == | ||
Revision as of 16:06, 21 May 2009
Contents |
The FpgNei Protein Superfamily
Functional Units
| G. Stereothermophilus Fpg | E. Coli Nei | ||||||||||||
|
|
| Functional Cluster | Variant 1 | Variant 2 | Fpg1 | Fpg2 | Plant | Neil1 | Neil2 | Neil3 | Proteo | Actino1 | Actino2 | MimiVirus |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Support for perfectly conserved Asn168 | Y | Y | N | N | N | N | N | N | N | N | ||
| Stability of catalytic helix | Y | Y | Y | N | N | N | N | N | N | N |