User:Lois A. Fridmann/Sandbox 3
From Proteopedia
| Line 42: | Line 42: | ||
6. VerPlank L, Bouamr F, LaGrassa T, Agresta B, Kikonyogo A, Leis J, Carter C. 2001. ''Tsg101, a homolgue of ubiquitin-conjugating (E2) enzymes, binds the L domain of HIV type 1 Pr55Gag.'' Proceeding of the National Academy of Science 98:7724 – 7729. | 6. VerPlank L, Bouamr F, LaGrassa T, Agresta B, Kikonyogo A, Leis J, Carter C. 2001. ''Tsg101, a homolgue of ubiquitin-conjugating (E2) enzymes, binds the L domain of HIV type 1 Pr55Gag.'' Proceeding of the National Academy of Science 98:7724 – 7729. | ||
| - | |||
| - | ==External Links== | ||
Revision as of 03:29, 6 June 2009
HIV-1 Gag Recruitment of Tsg101 and the Viral Budding Process
Contents |
Background and History
The Human Immunodefiency Virus (HIV) is the causative agent of acquired immunodefiency syndrome (AIDS) in which the immune system of a human begins to fail, leading to life threatening infections. HIV is a retrovirus that uses its host’s cellular machinery to replicate. The Tumor susceptibility gene 101 [Tsg101], is a human gene that encodes for a cellular protein, plays an important role in the pathogenesis of HIV and belongs to the ubiquitin-conjugating enzyme family, the ubiquitin E2 variant (UEV) subfamily.
In normal functioning cells, Tsg101, as a subunit of the ESCRT-1 (Endosomal Sorting Complex Required for Transport), promotes membrane alterations inside the cell that result in the formation of compartments (multivesicular bodies) in which proteins that have been marked for transport to the lysosome, through ubiquination, are sorted. Normally, a specific tetrapeptide PSAP binding motif on the endosomal protein Hrs (hepatocyte growth factor-regulated tyrosine kinase substrate) will bind to the specific P(S/T)AP binding pocket on the Tsg101 and deliver the protein cargo to endosomes that eventually become or fuse with lysosomes. If HIV is present in a cell, a major structural protein of the virus, Gag, recruits the Tsg101 protein using its own PTAP motif, which mimics the Hrs and binds to the binding pocket of the Tsg101 to gain access to the downstream machinery and to mediate viral budding as an alternative to degradation. The Gag protein is a Group-specific antigen and contains the genetic material that codes for core structural proteins of retroviruses. PTAP and PSAP motifs are able to bind Tsg101 equally.
Poly-ubiquitination inactivates Tsg101, possibly by altering the shuttling of a active membrane-bound protein with an inactive soluble protein.
| |||||||||
| 1m4p, 20 NMR models () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene: | tumor susceptibility gene 101 (Homo sapiens), Gag (Human immunodeficiency virus 1) | ||||||||
| Related: | 1kpp, 1kpq, 1m4q | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
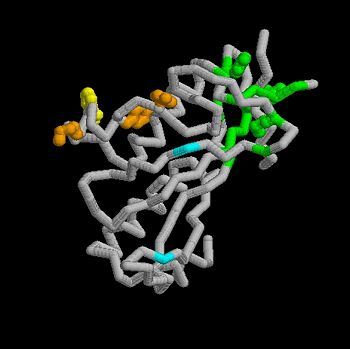
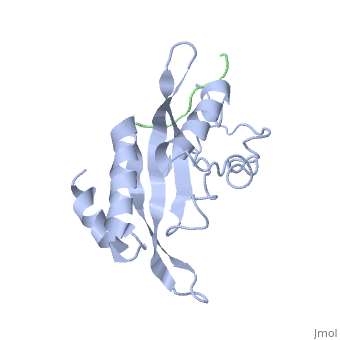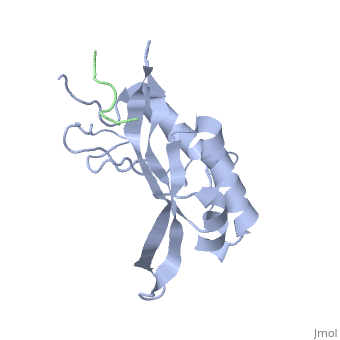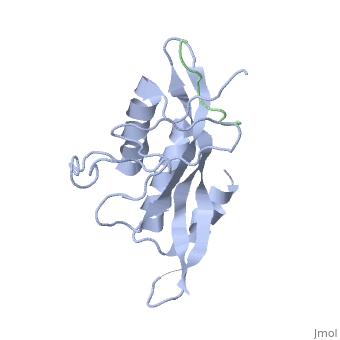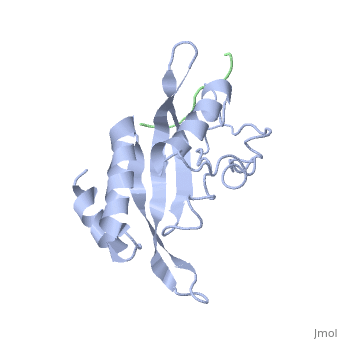
Structural and Functional Properties of the Tsg101
The specific HIV Tsg101 Ubiquitin E2 Variant (UEV) domain model represents the wild type version of the Tsg101 UEV domain with specific highlights of the PTAP binding site featured as a green backbone and three residues in the PTAP binding site (, , ), which are also colored green.. Four C-terminal domain residues , , colored orange and colored yellow, and two residues included in the Ubiquitin binding site featured as a cyan colored backbone (, ).
The three residues featured in the PTAP binding pocket exhibit potential areas where mutations can occur that results in the elimination or reduction of the HIV-1 Gag protein binding. The two residues featured in the Ubiquitin binding pocket feature potential areas where mutations can occur that inhibits Ubiquitination of the Tsg101 or the p6 region of the Gag binding to the PTAP binding pocket.
However, the main focus of the model is the C-terminal region of the UEV domain where the mutation of certain amino acid residues, which are not directly involved in the PTAP binding pocket result in the elimination or reduction of binding to the Gag protein. A mutation of W117 (yellow residues), which is conserved in all E2 enzymes, to A117 eliminates binding entirely. Mutations of Y110, Y113 and K118 (orange residues), which are unique to the Tsg101, to W110, V113 and A118 reduce binding by 31%, 33% and 50% respectively.
Mutations - PTAP binding pocket, ubiquitin binding pocket, C-terminal UEV Domain
Future Research and Therapies
Identification of mutations of various residues within and surrounding the PTAP binding site that inhibit HIV-1 Gag binding can help provide important information and avenues for future research on viral treatment and vaccination.
References
1. Liu F, Stephen Ag, Adamson C, Gousset K, Aman MJ, Freed EO, Fisher RJ, Burke TR, Jr. 2005. Hydrazone and Hydrazide-Containing N-Substituted Glycines as Peptoid Surrogates for Expedited Library Synthesis: Application to the Preparation of Tsg101-Directed HIV-1 Budding Antagonist. Org Lett 8: 5163-5168.
2. Macias M, Wiesnera S, Sudolb M. 2002. WW and SH3 domains, two different scaffolds to recognize proline-rich ligands. Protein Domains 513:30-37.
3. Pornillos O, Alam S, Davis D, Sundquist W. 2002. Structure of the Tsg101 UEV domain in complex with the PTAP motif of the HIV-1 p6 protein. Nature Structural Biology 9:812 – 817.
4. Pornillos O, Higginson D, Stray K, Fisher R, Garrus J, Payne M, He G, Wang H, Morham S, Sundquist W. 2003. HIV Gag mimics the Tsg101-recruiting activity of the human Hrs protein. The Journal of Cell Biology 162: 425-434.
5. Tavassoli A, Lu Q, Gam J, Pan H, Benkovic S, Cohen S. 2008. Inhibition of HIV Budding by a Genetically Selected Cyclic Peptide Targeting the Gag-TSG101 Interaction. American Chemical Society, Chemical Biology 3:757 - 764.
6. VerPlank L, Bouamr F, LaGrassa T, Agresta B, Kikonyogo A, Leis J, Carter C. 2001. Tsg101, a homolgue of ubiquitin-conjugating (E2) enzymes, binds the L domain of HIV type 1 Pr55Gag. Proceeding of the National Academy of Science 98:7724 – 7729.