Horse Liver Alcohol Dehydrogenase
From Proteopedia
| Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. Andrea Gorrell. |
Contents |
Horse Liver Alcohol Dehydrogenase
Meghan Hatcher
General Information
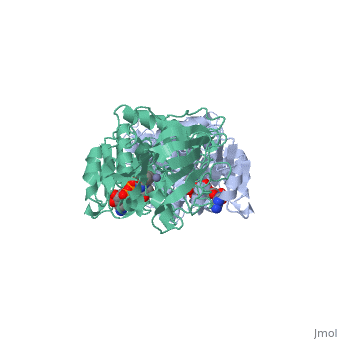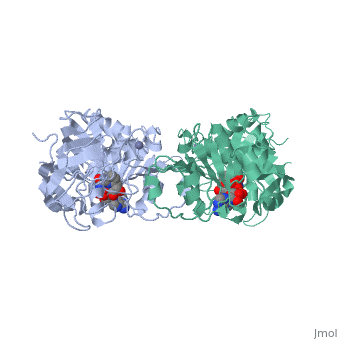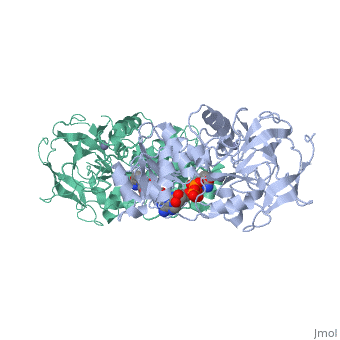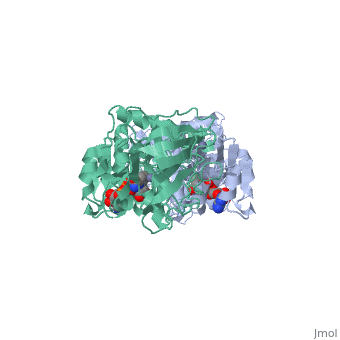
Alcohol dehydrogenase (LADH) is found in the liver of the species Equus Caballus (horse). It is a dimeric zinc-dependant protein, that catalyzes the reversible oxidation of primary and secondary alcohols to aldehyde, requiring the transfer of a hydride ion from the alcohol substrate to the cofactor nicotinamide adenine dinucleotide (NAD).
|
Protein Structure
Liver alcohol dehydrogenase (LADH)(1***) is a homodimeric protein, with each monomer having 374 amino acids and a mass of 40 kDa, each consisting of a catalytic and coenzyme binding domain. It is known that this protein contains two tryptophan residues per subunit, trp-15 and trp-314, and their location is well-known from the crystal structure of LADH and several of its derivatives and binary complexes. Insert sentence about here....
From structural determinants it by x-ray diffraction it is known that LADH undergoes conformational changes going from an open structure, in which large binding areas for coenzymes and substrates are accessible, towards a closed conformation. However the trigger mechanism for the structural transition is not yet fully understood. A complex pattern has emerged, showing that the structural changes in the LADH depend on coenzyme analogue structure and on which combination of coenzyme and a second ligand is present.
Protein Function
References
Proteopedia Page Contributors and Editors (what is this?)
Meghan Hatcher, Alexander Berchansky, David Canner, Michal Harel, Simmi Parhar, Andrea Gorrell, Eran Hodis