Adenylyl cyclase
From Proteopedia
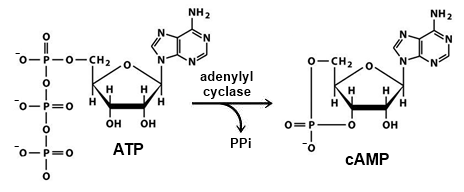
Template:STRUCTURE 1y11 Adenylyl cyclase (ADCY), also known as adenylate cyclase, is an enzyme which catalyzes the cyclization of adenosine triphosphate (ATP) into cyclic adenosine monophosphate (cAMP) which requires the cleavage of pyrophosphate (PPi)[1]. The cytoplasmic regions of ADCY consist of its N-terminal, C1a, C1b, C2a and C2b. The C1a and C2a make the catalytic domain. This page emphasizes on the microbial adenylyl cyclase Rv1264, but mammalian adenylyl cyclases are also covered in less detail.
Contents |
Introduction
There are ten isozymes of adenylyl cyclases in mammals, adenylyl cyclase type I-X, (ADCY I-X), and many more in other organisms[1]. All mammalian, and most other adenylyl cyclases belong to class III; most are integral membrane proteins, and all produce cAMP, the ability of which can be activated or inactivated in response to certain conditions or ligands[1]. All mammalian membrane bound adenylyl cyclases are activated by alpha subunits of G-proteins, but respond differently to ligands such as magnesium ions, calcium ions, and beta gamma subunits of G proteins[1]. One of the mammalian isozymes, and some prokaryotic forms of adenylyl cyclase respond to environmental conditions, primarily pH[2][3].
Reaction
Reactant
The reactant in the reaction catalyzed by adenylyl cyclase is ATP; ATP is the most abundant nucleotide triphosphate in most cells with typical concentrations ranging from 1 to 10mM[4]. This high intracellular concentration allows for cAMP concentrations to rise quickly in response to a specific signal, which is important in many signal transduction and metabolic pathways[5].
Reaction
The reaction occurs in a single, concerted step, where the oxygen on ATP's 3' hydroxyl group nucleophillically attacks the alpha-phosphate forming a phosphodiester bond and cleaving a pyrophosphate group[6]. In most active sites there is an acidic residue near the 3'OH which functions in its deprotonation, and basic residues by the β-phosphorous to lower the energy of the group for cleavage[7][8].
Products
The main product of this reaction is cAMP, with a side product of PPi[1].
Cyclic Adenosine Monophosphate
In mammals, cAMP acts as a secondary messenger, one of its functions is to control the activity of protein kinase A (PKA)[9]. In turn, PKA has quite diverse roles in cells, while most of them are associated with metabolism, PKA also plays important roles in transcription, the cell cycle, and apoptosis[9]. The ultimate fate of cAMP is its transformation into AMP by the cleavage of the phosphodiester bond by 3', 5'-cyclic adenosine monophosphate phosphodiesterase[9].
Pyrophosphate
Cleavage of the by-product of this reaction, PPi, by pyrophosphatase yields two molecules of inorganic phosphate (Pi)[10]. ATP synthase can reincorporate this inorganic phosphate into adenine diphosphate (ADP) to make ATP using energy in the proton motive force[11].
Mammalian Adenylyl Cyclase
There are ten isozymes of adenylyl cyclases in mammals, adenylyl cyclase type I-X, (ADCY I-X); In mammals adenylyl cyclase plays an important role in signal transduction pathways in which cAMP is a secondary messenger[12].
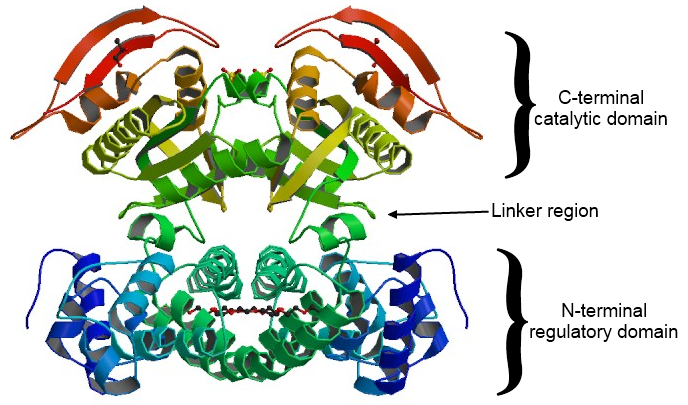
ADCY I-IX all share a general structure; They are composed of two trans-membrane regions (M1, M2) which are composed of six membrane-spanning helices and function to keep the enzyme anchored in the membrane, and two cytoplasmic regions (C1, C2) which can be further sub divided (C1a, C1b, C2a, C2b) and are responsible for all catalytic activity, and regulation by G-proteins and forskolin[12]. In solution, the C1a and C2a domains can form heterodimers with each other, either in the same or different enzymes, or they can form homodimers with their identical units on different enzymes[7]. The C1b domain is very large (≈15 kDa) with many regulatory sites, and has a variable structure across isozymes; while the C2b domain is nearly non-existent in many isozymes, and has yet to be associated with a particular function[7].
Type II
Structure
|
A monomer of C2 domain of type II adenylyl cyclase has an internal, hydrophobic, anti-parallel surrounded by several, amphipathic , except for an area which needed to form a homodimer with another C2 domain[7]. Two monomers of C2 domains of type II adenylyl cyclase bind together in solution to form a , which is necessary for catalytic conversion of ATP to cAMP and PPi[6]. When they are bound they create a deep crevasse spanning the center of their binding site; this crevasse is suited to bind two molecules at its ends[13]. Strong hydrogen bonds are made between oxygen atoms of forskolin and the surrounding peptide backbone, and the rest of the interactions are highly hydrophobic, as the forskolin binding site contains ten aliphatic and aromatic residues[13]. This binding of forskolin creates a hydrophobic linkage between the monomers, each of which has two different hydrophobic surfaces binding to forskolin; and it is this interaction which makes the homodimer stable[7][13]. The forskolin also interacts with and properly positions Asn 1025, which is essential for catalytic activity, and it may even interact directly with the ATP[13]. This homodimer-forskolin complex can be further activated in response to a signal via binding to a G-protein’s βγ-subunit [6][13]. This βγ-subunit binds to which make up part of an α-helix on the outermost layer of the complex[6][14].
The of this homodimer is located within the crevasse, and is characterized by two highly conserved sets of polar residues (Arg 997 (Green), Asn 1025 (Red), Ser1028(Pink), Arg 1029(Orange), Asp 1031(Yellow), and Ser 1032(Purple))[6][7]. One of these sets is located on each monomeric subunit, on the homodimer they arrange themselves in an anti-parallel fashion, where they point towards each other[7].
Over-expression Disorders
In the brain of mammals, the execution of memory based functions is carried out by the prefrontal cortex (PFC)[15]. Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels on neurons close to allow electrochemical signals to flow down the axon and into a synapse; when HCN channels are open, the electropotential signal cannot be transmitted through the cell[15]. Exposure of these channels to cAMP causes them open, stopping the transmission of signals, and thus impairs higher cognitive thoughts[15]. In patients with schizophrenia, a cAMP regulatory molecule, Disrupted-in-Schizophrenia 1 (DISC1) is mutated and cannot regulate cAMP levels[15]; Thus, elevated cAMP levels may cause schizophrenia. The closing of HCN channels are thought to play a part in other disorders, such as ADHD and bipolar disorder[15]. Thus, it is reasonable that regulation of cAMP production by targeting type II adenylyl cyclase, since it is found in the brain, may act as a treatment for these disorders.
Rv1264 Adenylyl Cyclase
Although adenylyl cyclase is found throughout organisms at a universal level, distantly related organisms have different modifications of the enzyme, each is specialized for a particular task in a particular environment[16]. As stated earlier humans have 10 known isozymes of adenylyl cyclase; whereas Escherichia coli has only one isozyme, and Mycobacterium tuberculosis has 15[16]. One particularly interesting adenylyl cyclase possessed by M. tuberculosis, Rv1264, has a N-terminal which, in a sense, acts as a pH sensor, as it regulates the activity of the enzyme based on the pH of the surrounding solution[3][16]. This adenylyl cyclase, like most others, belongs to class III, adenylyl cyclases in this class have multiple domains, at least one for catalysis, and another for regulation[16].
Structure
| |||||||||||
3D Structures of Adenylyl cyclase
2ev1, 2ev2, 2ev3, 2ev4 – MtADCY N-terminal – Mycobacterium tuberculosis
1y10, 1y11 – MtADCY holoenzyme
2fjt – ADCY 4 – Yersinia pestis
1ykd – ADCY – Anabaena
1ab8 – ADCY C2 domain - rat
ADCY catalytic domain
1yk9, 1ybt, 1ybu – MtADCY catalytic domain
1fx2, 1fx4 - ADCY catalytic domain – Trypanosoma brucei
2bw7 - SpADCY catalytic domain+catechol estrogen – Spirulina platensis
1wc0 – SpADCY catalytic domain+methylene ATP
1wc1, 1wc3, 1wc4, 1wc5, 1wc6 - SpADCY catalytic domain+ATP derivatives
3maa – dADCY 5 (mutant)+ADCY 2 C2a+guanine nucleotide binding protein G – dog
3g82 - dADCY 5 (mutant)+ADCY 2 C1a+guanine nucleotide binding protein G
3c14, 3c15 - dADCY 5 C1a (mutant)+ADCY 2 C2a+guanine nucleotide binding protein G+PPi+ions
3c16 - dADCY 5 C1a (mutant)+ADCY 2 C2a+guanine nucleotide binding protein G+ATP+Ca
2gvd, 2gvz - dADCY 5 C1a +ADCY 2 C2a+guanine nucleotide binding protein G+ATP+Mn
1tl7, 1u0h - dADCY 5 C1a +ADCY 2 C2a+guanine nucleotide binding protein G+GTP+Mn
1cs4 - dADCY 5 C1a +ADCY 2 C2a+guanine nucleotide binding protein G+AMP+Mg+PPI+GDP
1cul - dADCY 5 C1a +ADCY 2 C2a+guanine nucleotide binding protein G+GDP+Mg+triphosphate
1cjk - dADCY 5 C1a +ADCY 2 C2a+guanine nucleotide binding protein G+ATP+Mn+Mg
1cjt - dADCY 5 C1a +ADCY 2 C2a+guanine nucleotide binding protein G+GDP+Mn+Mg+ATP
1cju - dADCY 5 C1a +ADCY 2 C2a+guanine nucleotide binding protein G+GDP+Mg+ATP
1cjv - dADCY 5 C1a +ADCY 2 C2a+guanine nucleotide binding protein G+GDP+Mg+Zn+ATP
1azs - dADCY 5 C1a +ADCY 2 C2a+guanine nucleotide binding protein G+GDP+Mg
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Taussig R, Gilman AG. Mammalian membrane-bound adenylyl cyclases. J Biol Chem. 1995 Jan 6;270(1):1-4. PMID:7814360
- ↑ Pastor-Soler N, Beaulieu V, Litvin TN, Da Silva N, Chen Y, Brown D, Buck J, Levin LR, Breton S. Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J Biol Chem. 2003 Dec 5;278(49):49523-9. Epub 2003 Sep 25. PMID:14512417 doi:10.1074/jbc.M309543200
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Linder JU, Schultz A, Schultz JE. Adenylyl cyclase Rv1264 from Mycobacterium tuberculosis has an autoinhibitory N-terminal domain. J Biol Chem. 2002 May 3;277(18):15271-6. Epub 2002 Feb 11. PMID:11839758 doi:10.1074/jbc.M200235200
- ↑ Beis I, Newsholme EA. The contents of adenine nucleotides, phosphagens and some glycolytic intermediates in resting muscles from vertebrates and invertebrates. Biochem J. 1975 Oct;152(1):23-32. PMID:1212224
- ↑ Haring HU, Renner R, Hepp KD. Hormonal control of cyclic AMP turnover in isolated fat cells. Mol Cell Endocrinol. 1976 Aug-Sep;5(3-4):295-302. PMID:182581
- ↑ 6.0 6.1 6.2 6.3 6.4 Hurley JH. Structure, mechanism, and regulation of mammalian adenylyl cyclase. J Biol Chem. 1999 Mar 19;274(12):7599-602. PMID:10075642
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 Zhang G, Liu Y, Ruoho AE, Hurley JH. Structure of the adenylyl cyclase catalytic core. Nature. 1997 Mar 20;386(6622):247-53. PMID:9069282 doi:10.1038/386247a0
- ↑ Ketkar AD, Shenoy AR, Kesavulu MM, Visweswariah SS, Suguna K. Purification, crystallization and preliminary X-ray diffraction analysis of the catalytic domain of adenylyl cyclase Rv1625c from Mycobacterium tuberculosis. Acta Crystallogr D Biol Crystallogr. 2004 Feb;60(Pt 2):371-3. Epub 2004, Jan 23. PMID:14747729 doi:10.1107/S0907444903028002
- ↑ 9.0 9.1 9.2 Siddappa R, Martens A, Doorn J, Leusink A, Olivo C, Licht R, van Rijn L, Gaspar C, Fodde R, Janssen F, van Blitterswijk C, de Boer J. cAMP/PKA pathway activation in human mesenchymal stem cells in vitro results in robust bone formation in vivo. Proc Natl Acad Sci U S A. 2008 May 20;105(20):7281-6. Epub 2008 May 19. PMID:18490653
- ↑ Cox RP, Gilbert P Jr, Griffin MJ. Alkaline inorganic pyrophosphatase activity of mammalian-cell alkaline phosphatase. Biochem J. 1967 Oct;105(1):155-61. PMID:4964763
- ↑ Boyer PD. The ATP synthase--a splendid molecular machine. Annu Rev Biochem. 1997;66:717-49. PMID:9242922 doi:10.1146/annurev.biochem.66.1.717
- ↑ 12.0 12.1 Feinstein PG, Schrader KA, Bakalyar HA, Tang WJ, Krupinski J, Gilman AG, Reed RR. Molecular cloning and characterization of a Ca2+/calmodulin-insensitive adenylyl cyclase from rat brain. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10173-7. PMID:1719547
- ↑ 13.0 13.1 13.2 13.3 13.4 Tesmer JJ, Sunahara RK, Gilman AG, Sprang SR. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsalpha.GTPgammaS. Science. 1997 Dec 12;278(5345):1907-16. PMID:9417641
- ↑ Masters SB, Sullivan KA, Miller RT, Beiderman B, Lopez NG, Ramachandran J, Bourne HR. Carboxyl terminal domain of Gs alpha specifies coupling of receptors to stimulation of adenylyl cyclase. Science. 1988 Jul 22;241(4864):448-51. PMID:2899356
- ↑ 15.0 15.1 15.2 15.3 15.4 Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, Mazer JA, McCormick DA, Arnsten AF. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007 Apr 20;129(2):397-410. PMID:17448997 doi:10.1016/j.cell.2007.03.015
- ↑ 16.00 16.01 16.02 16.03 16.04 16.05 16.06 16.07 16.08 16.09 16.10 16.11 16.12 16.13 16.14 16.15 16.16 16.17 16.18 16.19 16.20 16.21 16.22 16.23 16.24 16.25 16.26 16.27 16.28 16.29 16.30 16.31 16.32 16.33 16.34 16.35 16.36 Tews I, Findeisen F, Sinning I, Schultz A, Schultz JE, Linder JU. The structure of a pH-sensing mycobacterial adenylyl cyclase holoenzyme. Science. 2005 May 13;308(5724):1020-3. PMID:15890882 doi:http://dx.doi.org/308/5724/1020
- ↑ 17.0 17.1 17.2 Tesmer JJ, Sunahara RK, Johnson RA, Gosselin G, Gilman AG, Sprang SR. Two-metal-Ion catalysis in adenylyl cyclase. Science. 1999 Jul 30;285(5428):756-60. PMID:10427002
- ↑ Tesmer JJ, Dessauer CW, Sunahara RK, Murray LD, Johnson RA, Gilman AG, Sprang SR. Molecular basis for P-site inhibition of adenylyl cyclase. Biochemistry. 2000 Nov 28;39(47):14464-71. PMID:11087399
- ↑ 19.0 19.1 19.2 19.3 19.4 19.5 19.6 19.7 19.8 Findeisen F, Linder JU, Schultz A, Schultz JE, Brugger B, Wieland F, Sinning I, Tews I. The structure of the regulatory domain of the adenylyl cyclase Rv1264 from Mycobacterium tuberculosis with bound oleic acid. J Mol Biol. 2007 Jun 22;369(5):1282-95. Epub 2007 Apr 12. PMID:17482646 doi:10.1016/j.jmb.2007.04.013
- ↑ Glickman MS, Jacobs WR Jr. Microbial pathogenesis of Mycobacterium tuberculosis: dawn of a discipline. Cell. 2001 Feb 23;104(4):477-85. PMID:11239406
- ↑ Channon JY, Kasper LH. Parasite subversion of the host cell endocytic network. Parasitol Today. 1995 Feb;11(2):47-8. PMID:15275372
- ↑ Warner DF, Mizrahi V. The survival kit of Mycobacterium tuberculosis. Nat Med. 2007 Mar;13(3):282-4. PMID:17342138 doi:10.1038/nm0307-282
- ↑ Lowrie DB, Jackett PS, Ratcliffe NA. Mycobacterium microti may protect itself from intracellular destruction by releasing cyclic AMP into phagosomes. Nature. 1975 Apr 17;254(5501):600-2. PMID:165421
- ↑ Rickman L, Scott C, Hunt DM, Hutchinson T, Menendez MC, Whalan R, Hinds J, Colston MJ, Green J, Buxton RS. A member of the cAMP receptor protein family of transcription regulators in Mycobacterium tuberculosis is required for virulence in mice and controls transcription of the rpfA gene coding for a resuscitation promoting factor. Mol Microbiol. 2005 Jun;56(5):1274-86. PMID:15882420 doi:10.1111/j.1365-2958.2005.04609.x
Proteopedia Page Contributors and Editors (what is this?)
Alexander Berchansky, Michal Harel, David Canner, Basavraj Khanppnavar, Travis Eyford, Joel L. Sussman