Semisynthetic Ribonuclease A
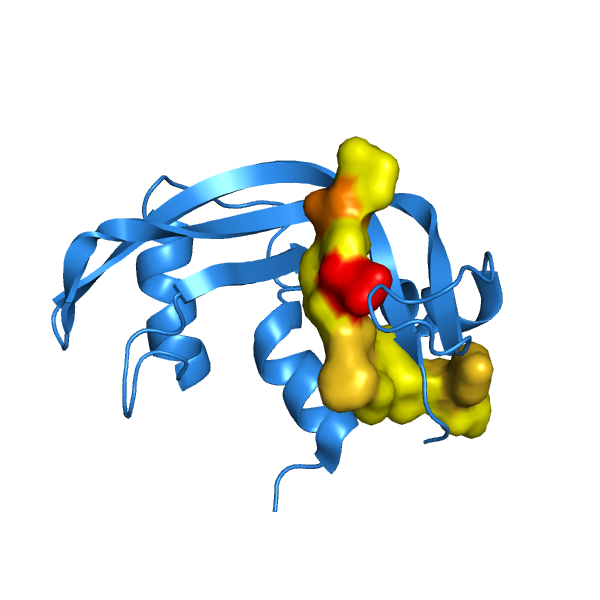
2-D Semisynthetic RNase A
Introduction
The synthesis of a fully active semisynthetic RNase A supports the hypothesis that the amino
acid sequence of a protein solely dictates the formation of an active enzyme and demonstrates
that an enzyme with the catalytic activity and specificity of a naturally produced enzyme can
be made in laboratory. Semisynthetic RNase A illustrates that functional enzymes can be produced
from merely the individual constituent amino acid residues. Polypeptide synthesis can provide
new routes to the study of enzymes through the selective modification of natural proteins to
assay individual roles of amino acids in folding and catalysis.
Function
The structure to function relationship is clearly exhibited by semisynthetic RNase A. In the
RNase A protein, the removal of six C terminal residues, leaving , completely halts enzymatic activity (Martin, 1987).
However, a complex of RNase 1-118 with a synthetic polypeptide comprising the
restores enzymatic activity to RNase A. Upon the addition of the synthetic chain, the semisynthetic
enzyme adopts a structure that closely resembles that of natural RNase (Martin, 1987). The
restoration of the structure reconstitutes the enzymatic activity of RNase to 98% (Martin, 1987).
Synthetic Method
The RNase 1-118 was prepared by successive digestion of RNase A pepsin and carboxypeptidase A
(Doscher, 1983). The synthetic component, RNase 111-124, was prepared by the use of solid-phase
peptide synthetic mothods, in which the peptide chain was assembled in the stepwise mannar while
it was attached at one end to a solid support. The peptide chain was extented by repetitive steps
of deprotection, neutralization and coupling until the desired sequence was obtained (Lin, 1970).
It was important that the synthesis proceeds rapidly and in high yields to prevent side reactions
or by-products.
Related Web-links
1. Introduction to Ribonuclease A by Raines:
http://www.uta.edu/faculty/sawasthi/Enzymology-4351-5324/Class%20Syllabus%20Enzymology/ribonucleaseA.pdf
2. Solid Phase Synthesis by Merrifield (Nobel Prize Winner):
http://nobelprize.org/nobel_prizes/chemistry/laureates/1984/merrifield-lecture.pdf
3. Chemical Synthesis of Proteins:http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2845543/?tool=pmcentrez
4. Refined Crystal Structure: http://www.ncbi.nlm.nih.gov/pubmed/3680234
References
Martin, Philip D., Marilynn S. Doscher, and Brian F. P. Edwards. "The Redefined Crystal Structure
of a Fully Active Semisynthetic Ribonuclease at 1.8-A Resolution." The Journal of Biological Chemistry
262.33 (1987): 15930-5938.
Marilynn S. Doscher, Philip D. Martin and Brian F.P. Edwards, "Characerization of the Histidine Proton
Nuclear Magnetic Resonance of a Semisynthetic Ribonuclease." Biochemistry, 1983,22,4125-4131.
Lin, M. C. (1970) Journal of Biological Chemistry, 245, 6726-6731.
David J. Boerema, Valentina. A. T., Stephen B. H. Kent, "Total Synthesis by Modern chemical Ligation
Methods and High Resolution (1.1-A) X-ray structure of Ribonuclease A. Biopolymers. 2008;90(3):278-86.
Link title

