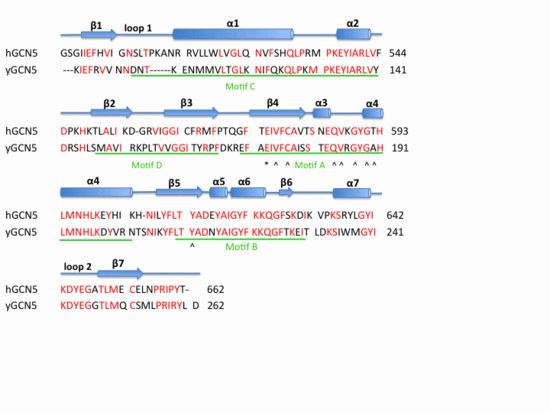
| Template:STRUCTURE 3d7c
Bromo Domain
The bromodomain is a highly conserved domain found to be a part of many chromatin remodeling proteins and nearly all HAT structures contain a bromodomain. This 110 amino acid motif was originally identified as a sequence motif common to the Drosophila brahma and female-sterile homeotic proteins, the yeast SWI2/SNF2 proteins and the human CCG1 protein [12][13] While the bromo domain is fairly close to the HAT domain of GCN5 no evidence suggests that it is involved in or necessary for histone acetyl transferase activity.
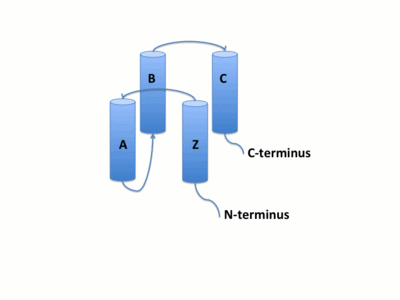
The GCN5 bromodomain is 71% α-helical domain of 110 amino acids with no β structures. The bromodoamin forms a consisting of helices αZ,αA,αB,and αC. In this four helix bundle the anti-parallel adjacent α helices pack together with angles approximately 18° to 20° to the helical axes. This arrangment of helices commonly found in helix bundles forms the "Ridges and Grooves" model. The model allow for the fitting of residues from one helix into the grooves of another. Additionally, the up-and-down four-helix bundle of the GCN5 bromodomain has left handed topology as a result of the orientation of the long ZA loop.
Weak Stabilizing Interactions II
Salt Bridge Formation is another example of weak interactions that can stabilize the secondary structures of proteins. The formation of salt bridges can increase packing density, increase hydrophobic core packing, and even decrease the length of surface loops.[14] [15]The formation of salt bridges involves both hydrogen bonding and electrostatic interactions. Salt bridges frequently occur between the anionic carboxylate of either Asp of Glu and cationic ammonium of Lys or Arg. Other residues with ionizable side chains, such as His, Tyr, and Ser may also participate in the formation of salt bridges. There are two ways in which to characterize salt bridge charge-charge interactions; where the cation and anion are hydrogen bonded to each other or the cation and anion are not hydrogen bonded to each other. The distance of salt bridges typically do not exceed 3.5 Å. The bromodomain has five involving residues 805, 822, 767, 770, 813, 816,828,831,780, and 776. One of these bridges involves the stabilization of helices αB and αC while another forms in the AZ Loop and may contribute the stabilization of the 3z helix.
Protein-Protein Interactions
Helices αZ and αA are connected by the ZA loop while helices αB and αC are connected by the BC loop. A two additional helices exist in the long ZA loop termed 3z and αA'. The ZA and BC loops pack together to form a hydrophobic pocket that may be involved in protein-protein interactions. [16] The is lined with residues Val757, Ala762, Tyr765, Tyr807, and Tyr814. The surface of this pocket is essentially the surface of the hydrophobic core of the bromodomain and some of the same residues that line this hydrophobic pocket are involved in the formation of the hydrophobic core. Residues that line the interior of the hydrophobic cavity include F753, V757, Y765, I773, D774, V800, N803, C804, Y807, N808, and Y814.
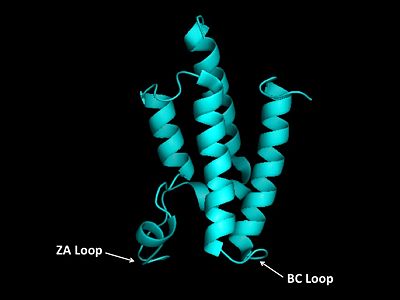 ZA and BC Loops of the bromo domain  Structural Predictions of PCAF_N Domain. 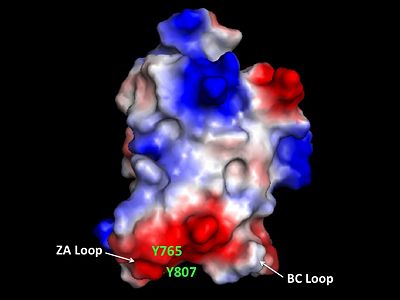 Electrostatic Map of hGCN5 bromodomain. 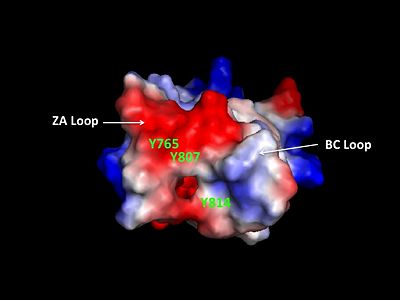 Hydrophobic pocket of the bromodomain. Three tyrosine residues line the hydrophobic pocket as a potential site for protein-protein interactions.
The bromodomain is separated from the HAT domain by 57 amino acids residues which contain an ADA2 interaction domain.[17]
Post-Translational Modifications
GCN5 contains one known site for posttranslational modification. A Try residue at position 734 is subject to phosphorylation by Tyrosine kinases. [18] [19] Residue Tyr734 is located in the helix of the bromodomain. The may act as a molecular switch that serves to activate or deactivate GCN5 and more specifically affect the bromodomain.
PCAF_N Domain
Structural Prediction
There is no known structure for the PCAF_N domain or p300/CBP assocaited factor N-terminal domain. From the known sequence for the PCAF_N domain, structural predictions can be generated for this domain. Predictprotein generates___________.The PCAF_N domain was predicted to be a mixed α+β structure. The secondary structural predictions indicated that the domain would consist of 31% helical, 4% beta strands, and 65% loop. Other important structural features that were predicted included one disulfide bond and three sites for postranslational modifications. Posttranslational modification sites included a N-glycosylation site, a Casein kinase II phosphorylation site, and a N-myristoylation site.
Taking advantage of Protein Homology/analogY Recognition Engine or PHYRE models for the PCAF_N domain can be generated.
http://www.sbg.bio.ic.ac.uk/phyre/html/index.html
https://www.predictprotein.org/
Evoultionary Conservation
Structural Homology
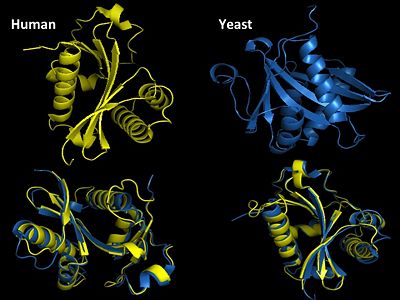 Structural Homology. Here is the HsGCN5(yellow) and yGCN5(blue) aligned HAT domains. 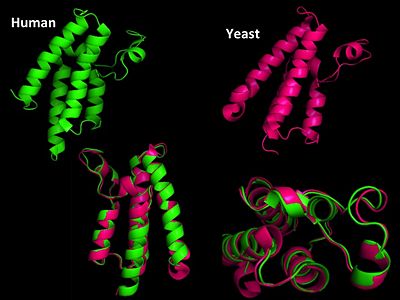 Structural Homology. Here is the HsGCN5(yellow) and yGCN5(blue) aligned HAT domains.
References
- ↑ Vetting MW, S de Carvalho LP, Yu M, Hegde SS, Magnet S, Roderick SL, Blanchard JS. Structure and functions of the GNAT superfamily of acetyltransferases. Arch Biochem Biophys. 2005 Jan 1;433(1):212-26. PMID:15581578 doi:10.1016/j.abb.2004.09.003
- ↑ Martinez E, Kundu TK, Fu J, Roeder RG. A human SPT3-TAFII31-GCN5-L acetylase complex distinct from transcription factor IID. J Biol Chem. 1998 Sep 11;273(37):23781-5. PMID:9726987
- ↑ Brand M, Yamamoto K, Staub A, Tora L. Identification of TATA-binding protein-free TAFII-containing complex subunits suggests a role in nucleosome acetylation and signal transduction. J Biol Chem. 1999 Jun 25;274(26):18285-9. PMID:10373431
- ↑ PMID: __________
- ↑ . PMID:5339769
- ↑ Wolffe AP, Hayes JJ. Chromatin disruption and modification. Nucleic Acids Res. 1999 Feb 1;27(3):711-20. PMID:9889264
- ↑ Schuetz A, Bernstein G, Dong A, Antoshenko T, Wu H, Loppnau P, Bochkarev A, Plotnikov AN. Crystal structure of a binary complex between human GCN5 histone acetyltransferase domain and acetyl coenzyme A. Proteins. 2007 Jul 1;68(1):403-7. PMID:17410582 doi:10.1002/prot.21407
- ↑ Clements A, Poux AN, Lo WS, Pillus L, Berger SL, Marmorstein R. Structural basis for histone and phosphohistone binding by the GCN5 histone acetyltransferase. Mol Cell. 2003 Aug;12(2):461-73. PMID:14536085
- ↑ Poux AN, Marmorstein R. Molecular basis for Gcn5/PCAF histone acetyltransferase selectivity for histone and nonhistone substrates. Biochemistry. 2003 Dec 16;42(49):14366-74. PMID:14661947 doi:10.1021/bi035632n
- ↑ Schuetz A, Bernstein G, Dong A, Antoshenko T, Wu H, Loppnau P, Bochkarev A, Plotnikov AN. Crystal structure of a binary complex between human GCN5 histone acetyltransferase domain and acetyl coenzyme A. Proteins. 2007 Jul 1;68(1):403-7. PMID:17410582 doi:10.1002/prot.21407
- ↑ Tanner KG, Langer MR, Kim Y, Denu JM. Kinetic mechanism of the histone acetyltransferase GCN5 from yeast. J Biol Chem. 2000 Jul 21;275(29):22048-55. PMID:10811654 doi:10.1074/jbc.M002893200
- ↑ Owen DJ, Ornaghi P, Yang JC, Lowe N, Evans PR, Ballario P, Neuhaus D, Filetici P, Travers AA. The structural basis for the recognition of acetylated histone H4 by the bromodomain of histone acetyltransferase gcn5p. EMBO J. 2000 Nov 15;19(22):6141-9. PMID:11080160 doi:10.1093/emboj/19.22.6141
- ↑ Tamkun JW, Deuring R, Scott MP, Kissinger M, Pattatucci AM, Kaufman TC, Kennison JA. brahma: a regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SNF2/SWI2. Cell. 1992 Feb 7;68(3):561-72. PMID:1346755
- ↑ Dhalluin C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou MM. Structure and ligand of a histone acetyltransferase bromodomain. Nature. 1999 Jun 3;399(6735):491-6. PMID:10365964 doi:10.1038/20974
- ↑ PMID: ___KS___
- ↑ Goldman A. How to make my blood boil. Structure. 1995 Dec 15;3(12):1277-9. PMID:8747452
- ↑ Candau R, Zhou JX, Allis CD, Berger SL. Histone acetyltransferase activity and interaction with ADA2 are critical for GCN5 function in vivo. EMBO J. 1997 Feb 3;16(3):555-65. PMID:9034338 doi:10.1093/emboj/16.3.555
- ↑ Rush J, Moritz A, Lee KA, Guo A, Goss VL, Spek EJ, Zhang H, Zha XM, Polakiewicz RD, Comb MJ. Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat Biotechnol. 2005 Jan;23(1):94-101. Epub 2004 Dec 12. PMID:15592455 doi:nbt1046
- ↑ Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, Hu Y, Tan Z, Stokes M, Sullivan L, Mitchell J, Wetzel R, Macneill J, Ren JM, Yuan J, Bakalarski CE, Villen J, Kornhauser JM, Smith B, Li D, Zhou X, Gygi SP, Gu TL, Polakiewicz RD, Rush J, Comb MJ. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007 Dec 14;131(6):1190-203. PMID:18083107 doi:S0092-8674(07)01522-X
|