Group:MUZIC:Zyxin
From Proteopedia

|
Contents |
ZYXIN
Zyxin is LIM domain protein of the zyxin/ajuba family, encode by the gene ZYX [1], that codes for a protein of 84 kDa that contains 3 LIM zinc-binding domains [1].
Function and structure
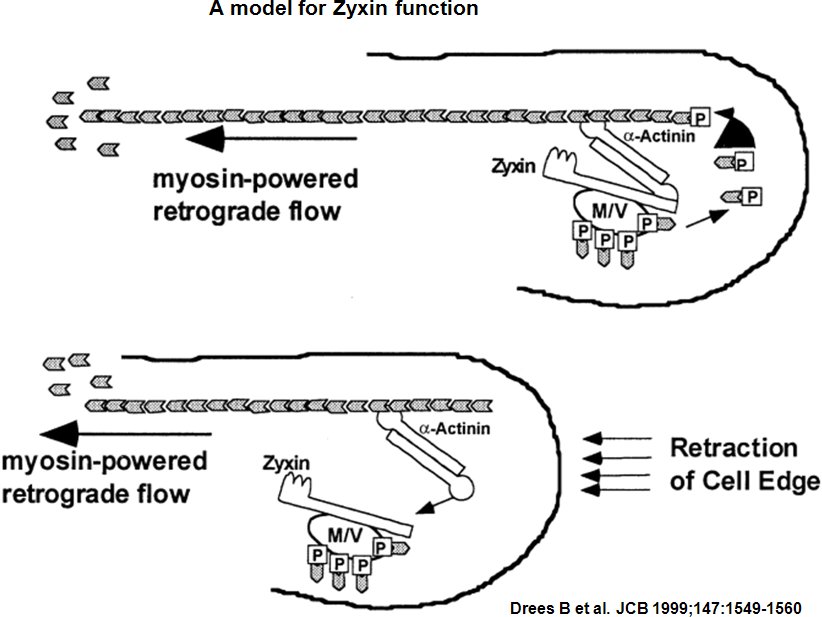
Zyxin is a zinc-binding phosphoprotein that concentrates at focal adhesions and along the actin cytoskeleton. Focal adhesions are actin-rich structures that enable cells to adhere to the extracellular matrix and at which protein complexes involved in signal transduction assemble. [2]. It is postulate, that Zyxin may be a component of a signal transduction pathway that mediates adhesion-stimulated changes in gene expression by similarity [3]. Zyxin has an N-terminal proline-rich domain and three LIM domains in its C-terminal half. The proline-rich domain may interact with SH3 domains of proteins involved in signal transduction pathways while the LIM domains are likely involved in protein-protein binding. Zyxin may function as a messenger in the signal transduction pathway that mediates adhesion-stimulated changes in gene expression and may modulate the cytoskeletal organization of actin bundles. Alternative splicing results in multiple transcript variants that encode the same isoform [4].
Interactions
Performing yeast two-hybrid analysis system, Yu and Luo found that myopodin interacts with zyxin both in vitro and in vivo and that this interaction leads to slower migration of prostate cancer cells and reduced invasiveness (Yu YP, Luo JH, 2006). Zyxin interacts with the E6 Protein from Human Papillomavirus Type 6 and this interaction results in its nuclear translocation [5]. Zyxin has been shown also to interact with ENAH [6], [7], LASP1 [8], LATS1 [9], Actinin, alpha 1 [10], [11] and Vasodilator-stimulated phosphoprotein [12].
References
- ↑ Sadler I, Crawford AW, Michelsen JW, Beckerle MC. Zyxin and cCRP: two interactive LIM domain proteins associated with the cytoskeleton. J Cell Biol. 1992 Dec;119(6):1573-87. PMID:1469049
- ↑ Macalma T, Otte J, Hensler ME, Bockholt SM, Louis HA, Kalff-Suske M, Grzeschik KH, von der Ahe D, Beckerle MC. Molecular characterization of human zyxin. J Biol Chem. 1996 Dec 6;271(49):31470-8. PMID:8940160
- ↑ Macalma T, Otte J, Hensler ME, Bockholt SM, Louis HA, Kalff-Suske M, Grzeschik KH, von der Ahe D, Beckerle MC. Molecular characterization of human zyxin. J Biol Chem. 1996 Dec 6;271(49):31470-8. PMID:8940160
- ↑ Gene ID: 7791
- ↑ Degenhardt YY, Silverstein S. Interaction of zyxin, a focal adhesion protein, with the e6 protein from human papillomavirus type 6 results in its nuclear translocation. J Virol. 2001 Dec;75(23):11791-802. PMID:11689660 doi:10.1128/JVI.75.23.11791-11802.2001
- ↑ Tani K, Sato S, Sukezane T, Kojima H, Hirose H, Hanafusa H, Shishido T. Abl interactor 1 promotes tyrosine 296 phosphorylation of mammalian enabled (Mena) by c-Abl kinase. J Biol Chem. 2003 Jun 13;278(24):21685-92. Epub 2003 Apr 2. PMID:12672821 doi:10.1074/jbc.M301447200
- ↑ Drees B, Friederich E, Fradelizi J, Louvard D, Beckerle MC, Golsteyn RM. Characterization of the interaction between zyxin and members of the Ena/vasodilator-stimulated phosphoprotein family of proteins. J Biol Chem. 2000 Jul 21;275(29):22503-11. PMID:10801818 doi:10.1074/jbc.M001698200
- ↑ Li B, Zhuang L, Trueb B. Zyxin interacts with the SH3 domains of the cytoskeletal proteins LIM-nebulette and Lasp-1. J Biol Chem. 2004 May 7;279(19):20401-10. Epub 2004 Mar 5. PMID:15004028 doi:10.1074/jbc.M310304200
- ↑ Hirota T, Morisaki T, Nishiyama Y, Marumoto T, Tada K, Hara T, Masuko N, Inagaki M, Hatakeyama K, Saya H. Zyxin, a regulator of actin filament assembly, targets the mitotic apparatus by interacting with h-warts/LATS1 tumor suppressor. J Cell Biol. 2000 May 29;149(5):1073-86. PMID:10831611
- ↑ Reinhard M, Zumbrunn J, Jaquemar D, Kuhn M, Walter U, Trueb B. An alpha-actinin binding site of zyxin is essential for subcellular zyxin localization and alpha-actinin recruitment. J Biol Chem. 1999 May 7;274(19):13410-8. PMID:10224105
- ↑ Li B, Trueb B. Analysis of the alpha-actinin/zyxin interaction. J Biol Chem. 2001 Sep 7;276(36):33328-35. Epub 2001 Jun 22. PMID:11423549 doi:10.1074/jbc.M100789200
- ↑ Harbeck B, Huttelmaier S, Schluter K, Jockusch BM, Illenberger S. Phosphorylation of the vasodilator-stimulated phosphoprotein regulates its interaction with actin. J Biol Chem. 2000 Oct 6;275(40):30817-25. PMID:10882740 doi:10.1074/jbc.M005066200