101d
From Proteopedia
|
REFINEMENT OF NETROPSIN BOUND TO DNA: BIAS AND FEEDBACK IN ELECTRON DENSITY MAP INTERPRETATION
Overview
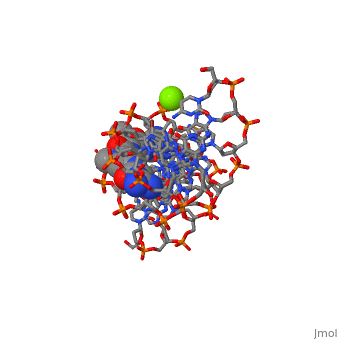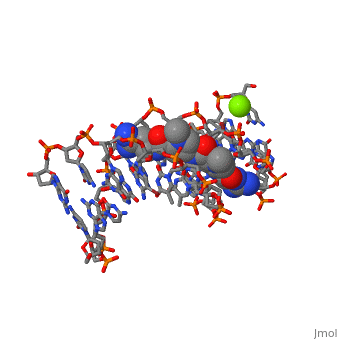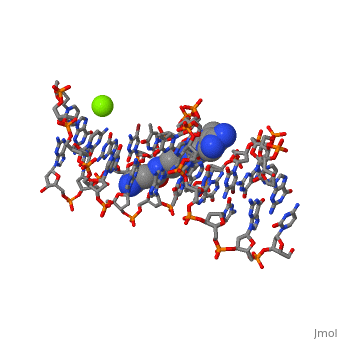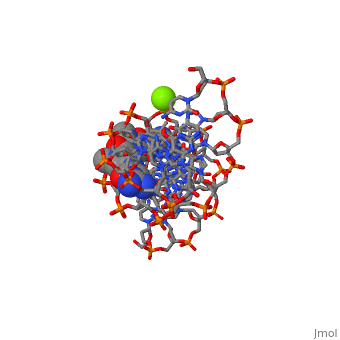
The X-ray crystal structure of the complex of the B-DNA dodecamer CGCGAATTCGCG with the antitumor drug netropsin has been reexamined to locate the drug accurately for computer-based drug design. The optimum solution is with the drug centered in the AATT region of the minor groove, making three good bifurcated hydrogen bonds with adenine N3 and thymine O2 atoms along the floor of the groove. Pyrrole rings of netropsin are packed against the C2 positions of adenines, leaving no room for the amine group of guanine and, hence, providing a structural rationale for the A.T specificity of netropsin. An alternative positioning in which the drug is shifted along the minor groove by ca. one-half base pair step is rejected on the basis of free R factor calculations and the appearance of the original drug-free difference maps. Final omit maps, although of more pleasing appearance, are not a dependable means of discriminating between right and wrong structures. The shifted alternative drug position ignores potential hydrogen bonding along the floor of the groove, provides no explanation for netropsin's observed A.T specificity, and is contradicted by NMR results [Patel, D. J. (1982) Proc. Natl. Acad. Sci. U.S.A. 79, 6424].
About this Structure
101D is a Protein complex structure of sequences from [1] with and as ligands. Full crystallographic information is available from OCA.
Reference
Refinement of netropsin bound to DNA: bias and feedback in electron density map interpretation., Goodsell DS, Kopka ML, Dickerson RE, Biochemistry. 1995 Apr 18;34(15):4983-93. PMID:7711020
Page seeded by OCA on Thu Feb 21 11:37:16 2008
Categories: Protein complex | Dickerson, R E. | Goodsell, D S. | Kopka, M L. | MG | NT | B-dna | Complexed with drug | Double helix | Modified