User:Joseph Whaley/Sandbox 650
From Proteopedia
|
Contents |
Introduction
Arginase belongs to the hydrolase family and is typically in the alpha-beta-alpha structure. The proteins associated with the hydrolase family play important roles in arginine metabolism, the urea cycle, and histidine degradation. This enzyme can be deficient in some organisms. Arginase deficiency is hereditary and rare. If arginase is missing, arginine is not broken down properly and urea cannot be produced; thus, nitrogen accumulates in the blood in the form of ammonia ultimately causing neurological and developmental issues. Arginase is the fifth and final enzyme used in the Urea Cycle to produce ornithine from arginine and exert ammonia as a biproduct. In order for arginase to to produce ornithine, the active site of arginine must be bound by a metal ion in order to facilitate hydrogen-bonding-assisted stability of the substrate. The enzyme demonstrates a highly specific active site allowing for a conserved structural arrangement allowing for the hydrolysis of arginine to form ornithine.
Structure and Functionality
Arginase is a part of the ureohydrolase enzyme family which is responsible for important roles in activities such as degradation and metabolism. It is mainly located in the liver and prostate system. This enzyme assists in transforming ammonia into urea by catalyzing the formation ofurea and ornithine from arginine.
Its structure includes a two-molecule metal cluster of , which can react with water stabilizing and orienting the molecule. Once this happens the water molecule is able to act as a nucleophile and attack arginie and transform it into urea and ornithine through hydrolysis. To be more descriptive, Arginase appears in the last reaction of the urea cycle. It catalyzes the reaction that hydrolytically cleaves the guanidinium group from the amino acid arginine. The active site containing the Manganese ions bind to a water molecule and form a nucleophilic hydroxide ion to attack the guanidinium carbon atom in arginine. Once ornithine is created it is then transported into the mitochondrion and continues through the urea cycle.
There are many types of Arginase enzymes but our main focus is Arginase-I which is part of the urea cycle.
Mechanism
The active site of Arginase holds arginine in place by the guanidinium group using hydrogen bonding. The guanidinium group hydrogen bonds with glutamate 227 (Glu227) setting it up for the nucleophilic attack by the hydroxide ion. This results in the creation of a tetrahedral intermediate. The manganese ion stabilize the sp3 lone electron pair forming on the ammonia (NH2) group and the hydroxide ion in the tetrahedral intermediate.
Arginase has a specific active site. The specificity is because of the high amount of hydrogen bonds between the enzyme and substrate. Water-faciliated hydrogen bonds saturate the four acceptor positions on the three positions on the alpha amino group and the alpha-carboxylate group. Inhibitors of arginase include 2(S)-amino-6-boronhexonic acid (ABH) and N-hydroxy-L-arginine (NOHA), which is an intermediate of nitric oxide biosynthesis.
Detailed mechanism: 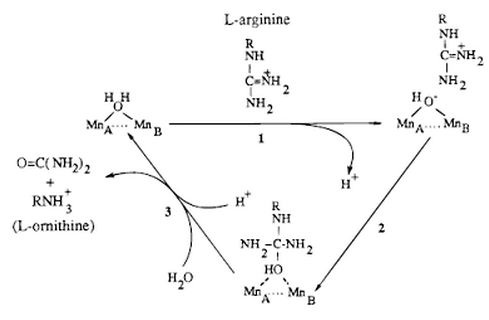
Implications and Applications
Agricultural Biotechnology
Current strategies exist in commercial agriculture in order to prevent crop loss by applying a chemical pesticide. Recent innovative technologies have been implemented in order to present transgenic crops with intrinsic pest resistance by producing plants with increased arginase I activity. In order to do this arginase is overproduced in plants to provide the plant with an enhanced resistance by acting as an anti-nutritive defense against herbivorous insects. This would be beneficial by reducing the need for chemical pesticides. Since arginase is present in all organisms, an increased production wouldn't be considered a foreign gene in genetically modified plants.
Arginase Regulation
References
^ MeSH Ureohydrolases ^ a b Lee J, Suh SW, Kim KH, Kim D, Yoon HJ, Kwon AR, Ahn HJ, Ha JY, Lee HH (2004). "Crystal structure of agmatinase reveals structural conservation and inhibition mechanism of the ureohydrolase superfamily". J. Biol. Chem. 279 (48): -. doi:10.1074/jbc.M409246200. PMID 15355972. ^ Christianson DW, Di Costanzo L, Sabio G, Mora A, Rodriguez PC, Ochoa AC, Centeno F (2005). "Crystal structure of human arginase I at 1.29-A resolution and exploration of inhibition in the immune response". Proc. Natl. Acad. Sci. U.S.A. 102 (37): -. doi:10.1073/pnas.0504027102. PMC 1201588. PMID 16141327. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1201588.
