Vascular Endothelial Growth Factor
From Proteopedia
| |||||||||||
|
Contents |
Structure of VEGF-E as a Model
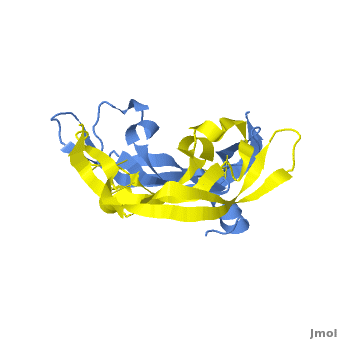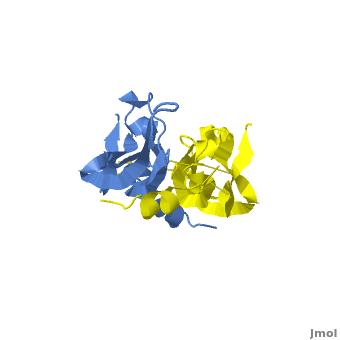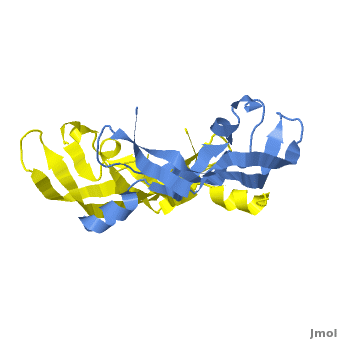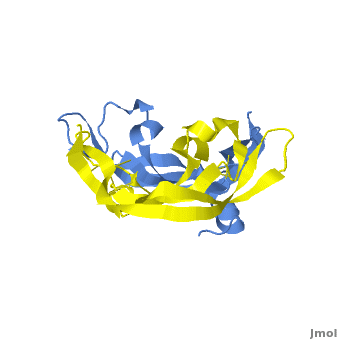
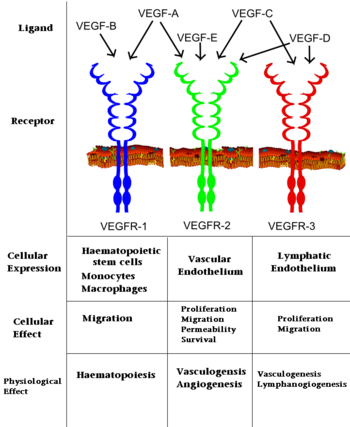
Although VEGF-E is only found in viral sources and thus is of less importance than VEGF-A, analysis of its structure is informative because it is the only member of the VEGF family that binds exclusively to VEGFR-2, the most essential VEGF receptor. Further, VEGF-E shares significant homology to VEGF-A, and thus can serve as an effective model. [12]
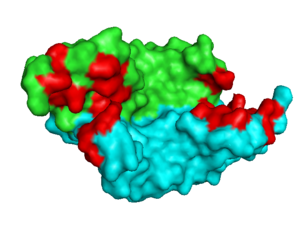
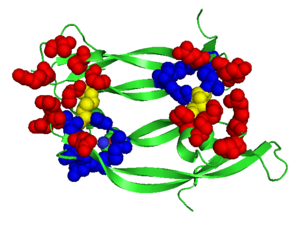
consists of a homodimer that is covalently linked by two intermolecular disulfide bonds between . Each monomer contains a central antiparallel beta sheet, with the canonical
found in other VEGFs. [13] The knot consists of an eight residue ring formed by the backbone of residues 57-61 and 102-104 and intramolecular disulfide bridges Cys57-Cys102 and Cys61-Cys104, and a third bridge, Cys26-Cys68, that passes perpendicularly through the ring. Each VEGF-E monomer contains an amino terminal alpha helix and three solvent accessible loop regions, L1, L2, and L3. are able to form a complex hydrogen bond network as well as extensive hydrophobic contacts with VEGFR making these loops ideal receptor specificity determinants. Residues: P34, S36, T43, P50, R46, D63, E64, and E67 make up the and are critical for binding to VEGFR-2 as determined by alanine mutagenesis.[14] Further, the salt bridge between is believed to be the source of VEGF-E’s VEGFR-2 specificity by preventing binding to VEGFR-1. [15]
Medical Implications
VEGF has received significant attention from the pharmaceutical industry in the hopes of correcting impaired vessel function. Such impaired vessel function accompanies many pathologies including atherosclerosis, arthritis, some neurodegenerative diseases such as ALS, and malignant cell growth such as cancerous tumors. [16] In fact, numerous studies highlighted the increased expression of VEGF in cancer cells resulting in tumoral angiogenesis, providing the tumor with the network of blood vessels need to grow and expand. VEGF expression is stimulated by hypoxia, a common characteristic of most newly formed tumors, and genetic mutations such as K-ras and p53, extremely common mutations present in a majority of cancers. [17] Since angiogenesis in typical adults is infrequent while extremely common in tumors, VEGF serves as a selective therapeutic target for cancer. A number of drugs, like Bevacizumab (a monoclonal antibody better known as Avastin) have been developed to interrupt the VEGF-VEGFR connection, with some success. Avastin binds to and inhibits all VEGF-A isoforms and has achieved megablockbuster status by earning over $5 billion in 2009 for Roche. [18]
3D Structures of VEGF
Updated February 2013
VEGF-A:
3bdy, 2qr0 - hVEGF-A + hFab light+heavy chain – human
2fjg, 2fjh - hVEGF-A receptor-binding domain+ hFab light+heavy chain
3p9w - hVEGF-A + hFab heavy chain
1tzh, 1tzi, 1cz8 - hVEGF-A + mFab YADS1 light+heavy chain - mouse
1mkg, 1mkk, 1mjv – hVEGF-A (mutant)
3qtk - hVEGF-A residues 34-135
4glu - hVEGF-A residues 1-102
1kmx - hVEGF-A heparin-binding domain
1vgh, 2vgh - hVEGF-A heparin-binding domain - NMR
1qty, 1flt – hVEGF-A receptor-binding domain + hFMS-like tyrosine
1vpp – hVEGF-A + receptor blocking peptide
1bj1 – hVEGF-A + mFab light+heavy chain fragments
2vpf, 1vpf – hVEGF-A receptor binding domain
1kat - hVEGF-A receptor binding domain + peptide antagonist - NMR
3s1b - hVEGF-A + Mini-Z
3s1k - hVEGF-A + Z-domain
4gln, 4gls - hVEGF-A + D-RFX001
VEGF-B
2xac – hVEGF-B receptor binding domain+ hVEGFR domain 2
2vwe – hVEGF-B + mFab light+heavy chain fragments
2c7w – hVEGF-B
VEGF-C
2x1w, 2x1x – hVEGF-C VEGF homology domain+ VEGFR-2 domains 2 & 3
VEGF-D
2xv7 – hVEGF-D VEGF homology domain
VEGF-F
1wq8, 1wq9 - VEGF-F – Vipera aspis
Additional Resources
For additional information, see: Cancer
See Also
References
- ↑ Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983 Feb 25;219(4587):983-5. PMID:6823562
- ↑ Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jun 15;161(2):851-8. PMID:2735925
- ↑ Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004 Aug;25(4):581-611. PMID:15294883 doi:10.1210/er.2003-0027
- ↑ Suto K, Yamazaki Y, Morita T, Mizuno H. Crystal structures of novel vascular endothelial growth factors (VEGF) from snake venoms: insight into selective VEGF binding to kinase insert domain-containing receptor but not to fms-like tyrosine kinase-1. J Biol Chem. 2005 Jan 21;280(3):2126-31. Epub 2004 Nov 12. PMID:15542594 doi:10.1074/jbc.M411395200
- ↑ Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992 Oct 29;359(6398):843-5. PMID:1279431 doi:http://dx.doi.org/10.1038/359843a0
- ↑ Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997 Feb;18(1):4-25. PMID:9034784
- ↑ Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996 Apr 4;380(6573):435-9. PMID:8602241 doi:http://dx.doi.org/10.1038/380435a0
- ↑ Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996 Apr 4;380(6573):435-9. PMID:8602241 doi:http://dx.doi.org/10.1038/380435a0
- ↑ Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001 Mar;114(Pt 5):853-65. PMID:11181169
- ↑ Muller YA, Li B, Christinger HW, Wells JA, Cunningham BC, de Vos AM. Vascular endothelial growth factor: crystal structure and functional mapping of the kinase domain receptor binding site. Proc Natl Acad Sci U S A. 1997 Jul 8;94(14):7192-7. PMID:9207067
- ↑ Keyt BA, Nguyen HV, Berleau LT, Duarte CM, Park J, Chen H, Ferrara N. Identification of vascular endothelial growth factor determinants for binding KDR and FLT-1 receptors. Generation of receptor-selective VEGF variants by site-directed mutagenesis. J Biol Chem. 1996 Mar 8;271(10):5638-46. PMID:8621427
- ↑ Pieren M, Prota AE, Ruch C, Kostrewa D, Wagner A, Biedermann K, Winkler FK, Ballmer-Hofer K. Crystal structure of the Orf virus NZ2 variant of vascular endothelial growth factor-E. Implications for receptor specificity. J Biol Chem. 2006 Jul 14;281(28):19578-87. Epub 2006 May 3. PMID:16672228 doi:10.1074/jbc.M601842200
- ↑ Oefner C, D'Arcy A, Winkler FK, Eggimann B, Hosang M. Crystal structure of human platelet-derived growth factor BB. EMBO J. 1992 Nov;11(11):3921-6. PMID:1396586
- ↑ Pieren M, Prota AE, Ruch C, Kostrewa D, Wagner A, Biedermann K, Winkler FK, Ballmer-Hofer K. Crystal structure of the Orf virus NZ2 variant of vascular endothelial growth factor-E. Implications for receptor specificity. J Biol Chem. 2006 Jul 14;281(28):19578-87. Epub 2006 May 3. PMID:16672228 doi:10.1074/jbc.M601842200
- ↑ Errico M, Riccioni T, Iyer S, Pisano C, Acharya KR, Persico MG, De Falco S. Identification of placenta growth factor determinants for binding and activation of Flt-1 receptor. J Biol Chem. 2004 Oct 15;279(42):43929-39. Epub 2004 Jul 21. PMID:15272021 doi:10.1074/jbc.M401418200
- ↑ Brockington A, Lewis C, Wharton S, Shaw PJ. Vascular endothelial growth factor and the nervous system. Neuropathol Appl Neurobiol. 2004 Oct;30(5):427-46. PMID:15488020 doi:10.1111/j.1365-2990.2004.00600.x
- ↑ Doyle B, Morton JP, Delaney DW, Ridgway RA, Wilkins JA, Sansom OJ. p53 mutation and loss have different effects on tumourigenesis in a novel mouse model of pleomorphic rhabdomyosarcoma. J Pathol. 2010 Jun 17. PMID:20662002 doi:10.1002/path.2748
- ↑ http://www.foxbusiness.com/story/markets/industries/technology/roche-increased-avastin-sales-efforts-doubles-force/
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, David Canner, Joel L. Sussman, Alexander Berchansky, Wayne Decatur, Jaime Prilusky