Structural highlights
Function
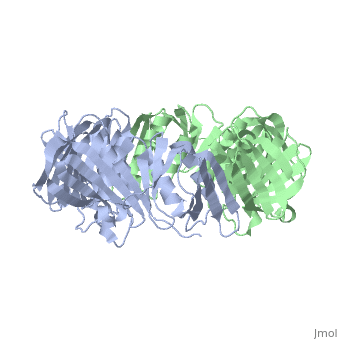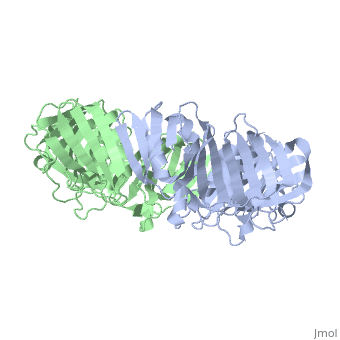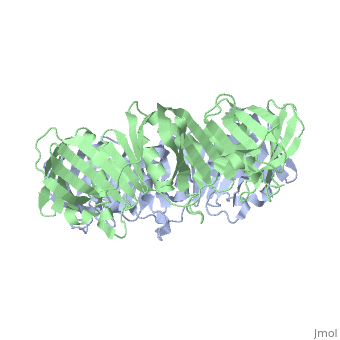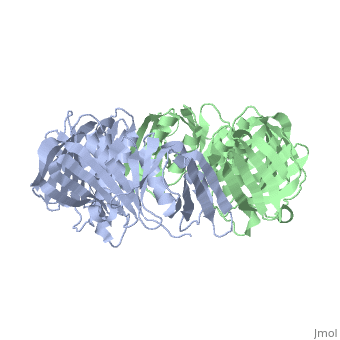
[DPO3B_ECOLI] DNA polymerase III is a complex, multichain enzyme responsible for most of the replicative synthesis in bacteria. This DNA polymerase also exhibits 3' to 5' exonuclease activity. The beta chain is required for initiation of replication once it is clamped onto DNA, it slides freely (bidirectional and ATP-independent) along duplex DNA.
Evolutionary Conservation
Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf.
Publication Abstract from PubMed
The crystal structure of the beta subunit (processivity factor) of DNA polymerase III holoenzyme has been determined at 2.5 A resolution. A dimer of the beta subunit (M(r) = 2 x 40.6 kd, 2 x 366 amino acid residues) forms a ring-shaped structure lined by 12 alpha helices that can encircle duplex DNA. The structure is highly symmetrical, with each monomer containing three domains of identical topology. The charge distribution and orientation of the helices indicate that the molecule functions by forming a tight clamp that can slide on DNA, as shown biochemically. A potential structural relationship is suggested between the beta subunit and proliferating cell nuclear antigen (PCNA, the eukaryotic polymerase delta [and epsilon] processivity factor), and the gene 45 protein of the bacteriophage T4 DNA polymerase.
Three-dimensional structure of the beta subunit of E. coli DNA polymerase III holoenzyme: a sliding DNA clamp.,Kong XP, Onrust R, O'Donnell M, Kuriyan J Cell. 1992 May 1;69(3):425-37. PMID:1349852[1]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Kong XP, Onrust R, O'Donnell M, Kuriyan J. Three-dimensional structure of the beta subunit of E. coli DNA polymerase III holoenzyme: a sliding DNA clamp. Cell. 1992 May 1;69(3):425-37. PMID:1349852