Sandbox Reserved 693
From Proteopedia
| This Sandbox is Reserved from 30/01/2013, through 30/12/2013 for use in the course "Biochemistry II" taught by Hannah Tims at the Messiah College. This reservation includes Sandbox Reserved 686 through Sandbox Reserved 700. |
To get started:
More help: Help:Editing |
6-Mercaptopurine activity and targeting
|
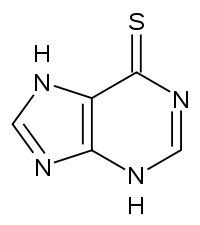
6-Mercaptopurine (6-MP) is an anti-cancer drug used for decades to treat acute lymphoblastic leukemia, and in recent years for other conditions such as inflammatory bowel disease. The drug is a purine derivative that resembles guanine, except that it has a thiol on the C6 of the base ring structure instead of a carbonyl, and is missing the amine on C2 (although this is later added before it is fully converted to 6-TGMP). Within the cell, 6-MP is converted to 6-thioguanosine monophosphate (6-TGMP) via hypoxanthine-guanine phosphoribosyltransferase (HGPRT) because it fits into the same active site as guanine, which is converted by the same enzyme to . Some of the 6-MP and 6-TGMP are deactivated by xanthine oxidase or thiopurine methyltransferase, but some of the 6-TGMP is able to inhibit cell division, attacking the cancer. It's interaction with HGPRT is analogous to the .
It can be seen in the above structure that human HGPRT has two identical subunits, shown in green and blue, each of which catalyzes one reaction. highlights the main structural motifs of the enzyme, with helices in green and sheets in blue.
|
After 6-MP is converted to 6-TGMP, it can be either inserted into a growing DNA strand which will cause problems with replication of the "infected" strand, or it can inhibit purine biosynthesis. This primarily happens at the enzyme glutamine phosophribosyl-pyrophosphate (PRPP) amidotransferase, which is used to start building the purine structure on a phosphorylated sugar. The can be seen here, with four subunits each shown in different colors.